This post continues my presentation of what’s new in the second edition of my book Computational Organic Chemistry. I present here a brief summary of the new materials in chapters 5-9. (See this previous post for what’s new in chapters 1-4.)
Every chapter has been updated, meaning that the topics from the First Edition that remain in this Second Edition (and that’s most of them) have been updated with any new relevant work that have appeared since 2007, when the First Edition was published. In addition, the following new subjects have been included.
Chapter 5. Diradicals and Carbenes
One of the major additions to the entire book appears in Chapter 5: the discovery of tunneling in a variety of carbenes. This work, pioneered by Schreiner and Allen, led to the discovery of tunneling control, a third means, in conjunction with thermodynamic control and kinetic control, for controlling product formation. This work is an exemplar of the synergy provided by experiments done in partnership with computations. The chapter also includes an interview with Prof. Peter Schreiner.
Chapter 6. Organic Reactions of Anions
The discussion on proline-catalyzed aldols includes many new computations, especially dealing with the possible intermediacy of oxazolidinones. A section on thiurea-catalyzed Claisen rearrangements, from the Jacobsen group, concludes the chapter, showing how the computational approaches to organocatalyzed reactions can be extended beyond the aldol and aldol-like reactions.
Chapter 7. Solution-Phase Organic Chemistry
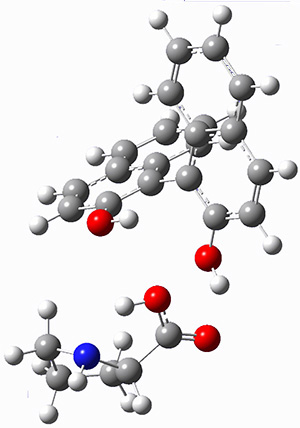
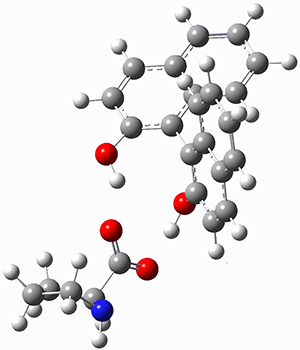
A discussion of solvent effects on amino acid structure has been added. This work focusses on the use of microsolvation to model local solvent effects, particularly in cases where proper accounting of strong hydrogen bonds can be critical in assessing behaviors.
Chapter 8. Organic Reaction Dynamics
A great deal of new materials appears in this chapter. Since the publication of the first edition of the book, many new studies have been published that greatly expand the types of organic reactions that are subject to dynamic effects. Of particular note are the many new examples of reactions on bifurcating surfaces. Some studies, principally by Singleton, now provide some guidance and hints towards predicting what types of reactions might exhibit non-statistical dynamics. Two new non-statistical dynamic types are presented: the roaming reactions and the roundabout mechanism in the SN2 reaction. The chapter ends with a detailed case study of the Wolff rearrangement.
Chapter 9. Computational Approaches to Understanding Enzymes
The last chapter is entirely new, and features how the techniques of computational organic chemistry, as discussed in the previous eight chapters, can be employed toward explicating enzymatic reactions. The chapter is not an in-depth survey of all of the activities in computational enzyme action – that would require its own full-length book – but rather it’s an overview to inspire you. The chapter begins with a brief discussion of enzymatic models, including the Pauling paradigm and Goodman’s model. Then computational strategies for addressing the large molecules involved in enzymatic studies are presented including QM/MM, adiabatic mapping, and the use of some very large-scale computations as benchmarks. Next, I present two case studies: of chorismate mutase and of catechol-O-methyltransferase (COMT). The chapter ends with a presentation of the progress in de novo design of enzymes capable of catalyzing specific reactions as developed by Baker and Houk.