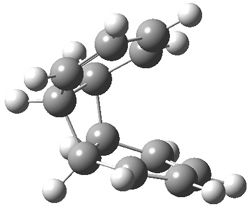
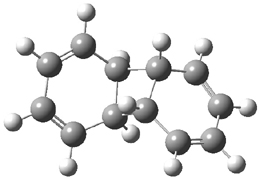
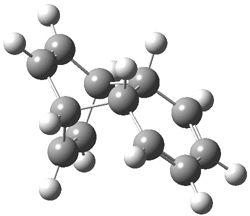
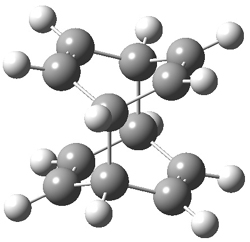
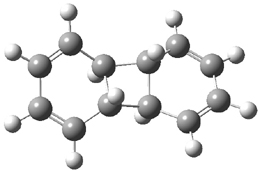
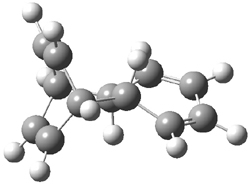
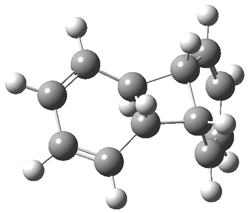
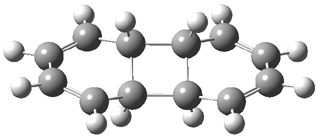
Hoffmann1 reports on a number of new benzene dimer structures, notably 5-8, whose RIJCOSX-MP2/cc-pVTZ2 structures are shown in Figure 1. A few of these new dimers are only somewhat higher in energy than the known dimers 1-4. The energies of these dimers, relative to two isolated benzene molecules, are listed in Table 1.
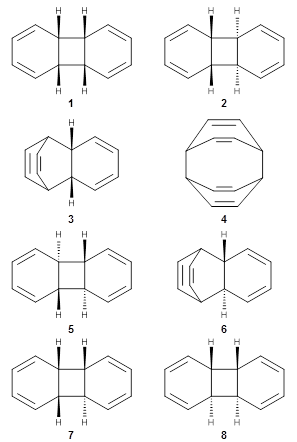
Figure 1. RIJCOSX-MP2/cc-pVTZ optimized geometries of 1-8.
Table 1. Energy (kcal mol-1) of the dimers relative to two benzene molecules and activation energy for reversion to two benzene molecules.
|
|
Compound
|
Erel
|
Eact
|
|
1
|
50.9
|
29
|
|
2
|
49.9
|
|
|
3
|
38.2
|
9
|
|
4
|
58.7
|
19
|
|
5
|
71.9
|
30
|
|
6
|
49.9
|
36
|
|
7
|
60.8
|
27
|
|
8
|
98.8
|
28
|
|
The energy for reversion of the isomers 5-8 to two isolated benzene molecules is calculated to be fairly large, and so they should be stable relative to that decomposition mode. They also examined a series of other decomposition modes, including [1,5]-hydrogen migration, all of which had barriers of 21 kcal mol-1 or greater, retrocyclization ([2+2]), for which they could not locate transition states, electrocyclic ring opening (Cope), with barriers of at least 17 kcal mol-1 and dimerization – some of which had relatively small enthalpic barriers of 4-5 kcal mol-1. However, the dimerizations all have very unfavorable entropic activation barriers.
So, the conclusion is that all of the novel dimers (4–8) can be reasonable expected to hang around for some time and therefore are potential synthetic targets.
References
(1) Rogachev, A. Yu.; Wen, X.-D.; Hoffmann, R. "Jailbreaking Benzene Dimers," J.
Am. Chem. Soc., 2012, 134, 8062-8065, DOI:10.1021/ja302597r
(2) Kossmann, S.; Neese, F. "Efficient Structure Optimization with Second-Order Many-Body Perturbation Theory: The RIJCOSX-MP2 Method," J. Chem. Theory Comput., 2010, 6, 2325-2338, DOI: 10.1021/ct100199k
InChIs
1: InChI=1S/C12H12/c1-2-6-10-9(5-1)11-7-3-4-8-12(10)11/h1-12H/t9-,10+,11-,12+
InChIKey=WMPWOGVJEXSFLI-UHFFFAOYSA-N
2: InChI=1S/C12H12/c1-2-6-10-9(5-1)11-7-3-4-8-12(10)11/h1-12H/t9-,10+,11+,12-
InChIKey=WMPWOGVJEXSFLI-IWDIQUIJSA-N
3: InChI=1S/C12H12/c1-2-4-12-10-7-5-9(6-8-10)11(12)3-1/h1-12H/t9?,10?,11-,12+
InChIKey=ONVDJSCNMCYFTI-CAODYFQJSA-N
4: InChI=1S/C12H12/c1-2-10-4-3-9(1)11-5-7-12(10)8-6-11/h1-12H
InChIKey=BCBHEUOKKNYIAT-UHFFFAOYSA-N
5: InChI=1S/C12H12/c1-2-6-10-9(5-1)11-7-3-4-8-12(10)11/h1-12H/t9-,10-,11+,12+/m1/s1
InChIKey=WMPWOGVJEXSFLI-WYUUTHIRSA-N
6: InChI=1S/C12H12/c1-2-4-12-10-7-5-9(6-8-10)11(12)3-1/h1-12H/t9?,10?,11-,12-/m0/s1
InChIKey=ONVDJSCNMCYFTI-QQFIATSDSA-N
7: InChI=1S/C12H12/c1-2-6-10-9(5-1)11-7-3-4-8-12(10)11/h1-12H/t9-,10-,11-,12+/m1/s1
InChIKey=WMPWOGVJEXSFLI-KKOKHZNYSA-N
8: InChI=1S/C12H12/c1-2-6-10-9(5-1)11-7-3-4-8-12(10)11/h1-12H/t9-,10-,11-,12-
InChIKey=WMPWOGVJEXSFLI-NQYKUJLISA-N