The concept of complementarity between enzyme and substrate, especially the transition state for reactions at the substrate, is a key element of Pauling’s model for enzymatic activity. Koshland’s “induced fit” modification suggests that the enzyme might change its structure during the binding process to either destabilize the reactant or help stabilize the TS. These concepts are now tested in a very nice model by Stoddart, Siegel and coworkers.1
Stoddart recently reported the host compound ExBox4+ 1 and demonstrated that it binds planar polycyclic aromatic hydrocarbons.2 (I subsequently reported DFT computations on this binding.) The twist in this new paper is the binding of corranulene 2 inside ExBox4+ 1. Corranulene is bowl-shaped, with a bowl inversion barrier of 11.5 kcal mol-1 (10.92 kcal mol-1 at B97D/Def2-TZVPP).
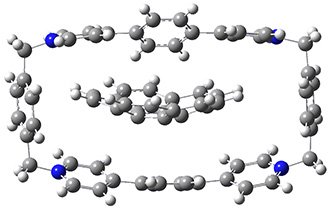
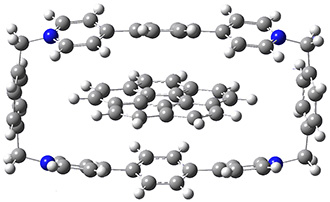
The corranulene bowl is too big to fit directly into 1 without some distortions. The x-ray structure of the complex of 1 with 2 inside shows the width of 1 expanding by 0.87 Å and the bowl depth of 2 decreasing by 0.03 Å. The B97D/Def2-TZVPP optimized geometry of this complex (shown in Figure 1) shows similar distortions – the width of 1 increases by 0.37 Å (gas) or 0.29 Å (acetone solution), while the bowl depth of 2 decreases by 0.03 Å (gas) or 0.02 Å (solution).
|
ground state |
|
transition state |
Figure 1. B97D/Def2-TZVPP optimized geometries of the complex of 2 inside 1 (a) ground state and (b) transition state.
The calculated structure of the bowl inversion transition state of 2 inside of 1 is shown in Figure 1. 2 is planar at the TS. The experimental inversion barrier (determined by variable temperature NMR line shift analysis) is 7.88 kcal mol-1, while the calculated barrier is 8.77 kcal mol-1. The reduction in the bowl inversion barrier of 2 inside of 1 is therefore about 2.5 kcal mol-1. The authors argue that this barrier reduction can be attributed to about 0.5 kcal mol-1 of destabilization of the ground state of 2 along with 2 kcal mol-1 of stabilization of the transition state afforded by the host. This study thus confirms the notions of a host reducing a barrier (through both transition state stabilization and ground state destabilization) and induced fit.
References
(1) Juríček, M.; Strutt, N. L.; Barnes, J. C.; Butterfield, A. M.; Dale, E. J.; Baldridge, K. K.; Stoddart, J. F.; Siegel, J. S. "Induced-fit catalysis of corannulene bowl-to-bowl inversion," Nat. Chem. 2014, 6, 222-228, DOI: 10.1038/nchem.1842.
(2) Barnes, J. C.; Juríček, M.; Strutt, N. L.; Frasconi, M.; Sampath, S.; Giesener, M. A.; McGrier, P. L.; Bruns, C. J.; Stern, C. L.; Sarjeant, A. A.; Stoddart, J. F. "ExBox: A Polycyclic Aromatic Hydrocarbon Scavenger," J. Am. Chem. Soc. 2012, 135, 183-192, DOI: 10.1021/ja307360n.
(3) Bachrach, S. M. "DFT Study of the ExBox·Aromatic Hydrocarbon Host–Guest Complex," J. Phys. Chem. A 2013, 117, 8484-8491, DOI: 10.1021/jp406823t.
InChIs
1: InChI=1S/C48H40N4/c1-2-38-4-3-37(1)33-49-25-17-45(18-26-49)41-9-11-43(12-10-41)47-21-29-51(30-22-47)35-39-5-7-40(8-6-39)36-52-31-23-48(24-32-52)44-15-13-42(14-16-44)46-19-27-50(34-38)28-20-46/h1-32H,33-36H2/q+4
InChIKey=ZMELWAYDWQWNOQ-UHFFFAOYSA-N
2: InChI=1S/C20H10/c1-2-12-5-6-14-9-10-15-8-7-13-4-3-11(1)16-17(12)19(14)20(15)18(13)16/h1-10H
InChIKey=VXRUJZQPKRBJKH-UHFFFAOYSA-N