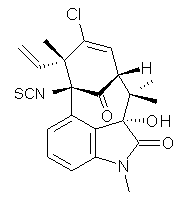
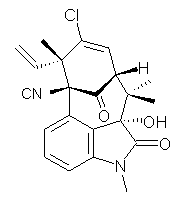
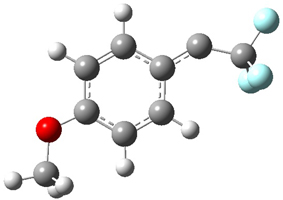
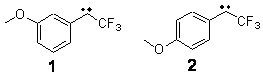A quick note here on the use of computed NMR to determine stereochemical structure. The Garg group synthesized two “oxidized welwitindolines”, compounds 1 and 2.1 The relative stereochemistry at the C3 position (the carbon with the hydroxy group) was unknown.
|
|
|
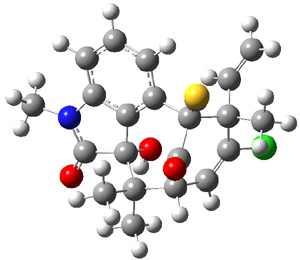
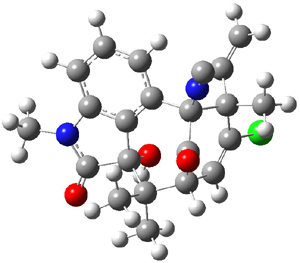
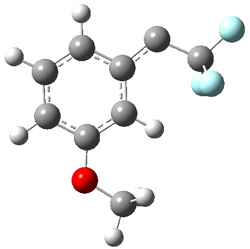
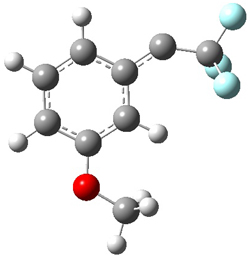
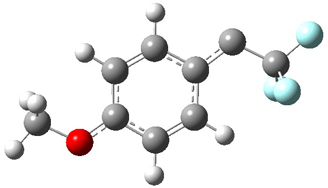
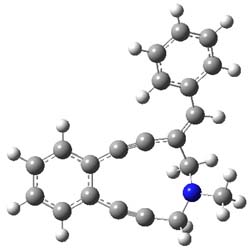
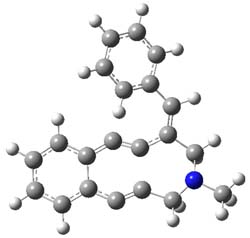
Low energy gas-phase conformers of both epimers of 1 and 2 were optimized at B3LYP/6-31+G(d,p). (These computations were done by the Tantillo group.) See Figure 1 for the optimized lowest energy conformers. Using these geometries the NMR chemical shifts were computed at mPW1PW91/6-311+G(d,p) with implicit solvent (chloroform). The chemical shifts were Boltzmann-weighted and scaled according to the prescription (see this post) of Jain, Bally and Rablen.2 The computed chemical shifts were then compared against the experimental NMR spectra. For both 1 and 2, the 13C NMR shifts could not readily distinguish the two epimers. However, the computed 1H chemical shifts for the S epimer of each compound was significantly in better agreement with the experimental values; the mean average deviation for the S epimer of 2 is 0.08 ppm but 0.36ppm for the R epimer. As a check of these results, DP4 analysis3 (see this post) of 2 indicated a 100% probability for the S epimer using only the proton chemical shifts or with the combination of proton and carbon data.
|
1 |
2 |
Figure 1. B3LYP/6-31+G(d,p) optimized geometries of the
lowest energy conformations of 1 and 2.
References
(1) Quasdorf, K. W.; Huters, A. D.; Lodewyk, M. W.; Tantillo, D. J.; Garg, N. K., "Total Synthesis of Oxidized Welwitindolinones and (-)-N-Methylwelwitindolinone C Isonitrile," J. Am. Chem. Soc. 2011, 134, 1396-1399, DOI: 10.1021/ja210837b
(2) Jain, R.; Bally, T.; Rablen, P. R., "Calculating Accurate Proton Chemical Shifts of Organic Molecules with Density Functional Methods and Modest Basis Sets," J. Org. Chem. 2009, 74, 4017-4023, DOI: 10.1021/jo900482q.
(3) Smith, S. G.; Goodman, J. M., "Assigning Stereochemistry to Single Diastereoisomers by GIAO NMR Calculation: The DP4 Probability," J. Am. Chem. Soc. 2010, 132, 12946-12959, DOI: 10.1021/ja105035r
InChIs
1: InChI=1/C22H21ClN2O3S/c1-6-20(4)15(23)10-13-17(26)21(20,24-11-29)12-8-7-9-14-16(12)22(28,19(13,2)3)18(27)25(14)5/h6-10,13,28H,1H2,2-5H3/t13-,20+,21+,22-/m0/s1
InChIKey=VDNAFECSPBXWIH-WOHBTAIZBQ
2: InChI=1/C22H21ClN2O3/c1-7-20(4)15(23)11-13-17(26)21(20,24-5)12-9-8-10-14-16(12)22(28,19(13,2)3)18(27)25(14)6/h7-11,13,28H,1H2,2-4,6H3/t13-,20+,21+,22-/m0/s1
InChIKey=GFNPBZSGZFQTJA-WOHBTAIZBX