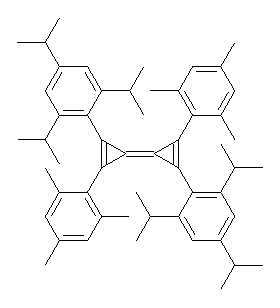
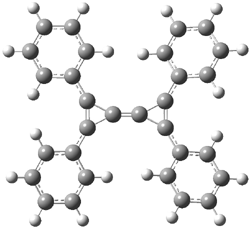
Here’s another great example of synthesis of highly strained compounds. Bertrand has prepared the substituted triafulvalene 1.1 The compound is stable as a solid or in solution under inert gas. It does however react quickly with water, a remarkable addition of water across an alkene. This is understood in terms of a very high HOMO and a low LUMO, indicating a very reactive double bond. The UV/Vis corroborates this: its absorption is at 502nm, compared to 171nm of ethylene and 217nm of 1,3-butadiene. The B3LYP/6-31G(d) structure of the tetraphenyl derivative 2 is shown in Figure 1.
|
|
|
2 |
Figure 1. B3LYP/6-31G(d) optimized structure of 2.
References
(1) Kinjo, R.; Ishida, Y.; Donnadieu, B.; Bertrand, G., "Isolation of Bicyclopropenylidenes: Derivatives of the Smallest Member of the Fulvalene Family," Angew. Chem. Int. Ed. 2009, 48, 517-520, DOI: 10.1002/anie.200804372
InChIs
1: InChIKey=GJHAFFXCMBMUNM-DBFBYELTBP
2: InChIKey=WTGGHSXPMAHUNP-UHFFFAOYAY