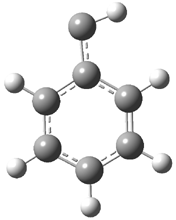
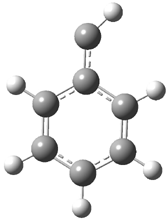
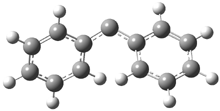
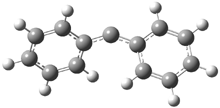
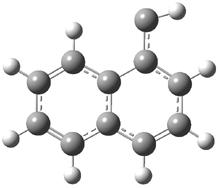
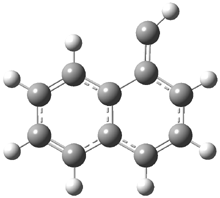
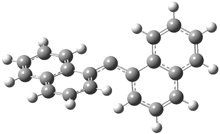
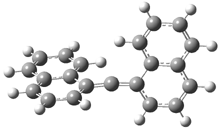
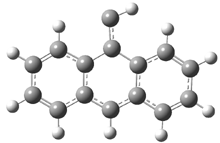
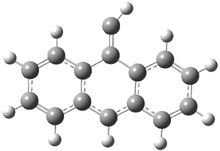
In the book I extensively discuss the singlet-triplet gap of methylene and some of the chemistry of phenylcarbene. Schleyer and Schaefer have now reported computations on the singlet-triplet gap of arylcarbenes.1 The geometries of phenylcarbene 1, diphenylcarbene 2, 1-naphthylcarbene 3, bis(1-naphtyl)carbene 4, and 9-anthrylcarbene 5 were optimized at B3LYP/6-311+G(d,p). These geometries are shown in Figure 1.

|
1s |
1t |
|
2s |
2t |
|
3s |
3t |
|
4s |
4s |
|
4s |
4s |
Figure 1. B3LYP/6-311+G(d,p) optimized structures of singlet and triplet 1-5.
Since this functional is known to underestimate the singlet-triplet gap of carbenes, they employ an empirical correction based on the difference in this gap for methylene between the computed value (11.89 kcal mol-1) and the experimental value (9.05 kcal mol-1). These corrected energy gaps are listed in Table 1.
Table 1. Corrected singlet-triplet energy gaps (kcal mol-1) at B3LYP/6-311+G(d,p).
|
Molecule |
ΔEST |
|
1 |
2.75 |
|
2 |
2.94 |
|
3 |
3.40 |
|
4 |
3.74 |
|
5 |
5.67 |
Using the following isodesmic reactions, they estimate the stabilization of the singlet or triplet carbene afforded by the aryl substituent:
R-C-H + CH4 → H-C-H + R-CH3
R-C-R + CH4 → R-C-H + R-CH3
These isodesmic energies are listed in Table 2. For phenylcarbne, the phenyl group stabilizes the singlet more than the triple, reducing the ST gap by 6.3 kcal mol-1. However, adding a second phenyl group (making 2) stabilizes both the singlet and triplet by about the same amount, leading to little change in the ST gap. The singlet does not get accrue the potential benefit of the second aryl group because sterics prohibit the two rings from being coplanar.
Table 2. Aryl effect for 1-5 based on the isodesmic reaction energies (kcal mol-1)
|
|
||
|
Molecule |
ΔEsinglet |
ΔEtriplet |
|
1 |
24.4 |
18.1 |
|
2 |
15.8 |
16.0 |
|
3 |
26.6 |
20.9 |
|
4 |
18.6 |
19.0 |
|
5 |
30.5 |
26.8 |
|
|
||
References
(1) Woodcock, H. L.; Moran, D.; Brooks, B. R.; Schleyer, P. v. R.; Schaefer, H. F., "Carbene Stabilization by Aryl Substituents. Is Bigger Better?," J. Am. Chem. Soc., 2007, 129, 3763-3770, DOI: 10.1021/ja068899t.
InChIs
1: InChI=1/C7H6/c1-7-5-3-2-4-6-7/h1-6H
2: InChI=1/C13H10/c1-3-7-12(8-4-1)11-13-9-5-2-6-10-13/h1-10H
3: InChI=1/C11H8/c1-9-5-4-7-10-6-2-3-8-11(9)10/h1-8H
4: InChI=1/C21H14/c1-2-8-19-14-16(12-13-17(19)6-1)15-20-10-5-9-18-7-3-4-11-21(18)20/h1-14H
5: InChI=1/C15H10/c1-11-14-8-4-2-6-12(14)10-13-7-3-5-9-15(11)13/h1-10H