Optical activity is a major tool for identifying enantiomers. With recent developments in computational techniques, it is hoped that experiments combined with computations will be a powerful tool for determining absolute configuration. The recent work of Lahiri, et al. demonstrates the scope of theoretical work that is still needed to really make this approach broadly applicable.1
They prepared (1R,4R)-norbornenone 1 and measured its optical rotation in the gas phase and in dilute solutions of acetonitrile and cyclohexane. The specific rotations at three different wavelengths are listed in Table 1. Of first note is that though there is some small differences in solution, as expected, there really is substantial differences between the gas- and solution phases. Thus cautionary point 1: be very careful of comparing solution phase experimental optical activity with computed gas phase predictions.
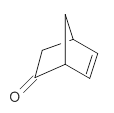
1
Table 1. Experimental and computed specific rotation of 1.
|
|
355.0 nm |
589.3 nm |
633.0 nm |
|
Gas phase |
|||
|
Expt |
6310 |
755 |
617 |
|
B3LYP |
10887 |
1159 |
944 |
|
CCSD |
3716 |
550 |
453 |
|
Acetonitrile solution |
|||
|
Expt |
8607 |
950 |
776 |
|
PCM/B3LYP |
11742 |
1277 |
1040 |
|
Cyclohexane solution |
|||
|
Expt |
9159 |
981 |
799 |
|
PCM/B3LYP |
11953 |
1311 |
1069 |
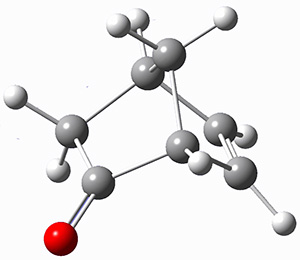
For the computations, the geometry of 1 was optimized at B3LYP/aug-cc-pVTZ (see Figure 1. The OR was computed at B3LYP with different basis sets, finding that the difference in the predicted specific rotation at 598.3nm differs only quite little (90.6 deg dm-1 (g/mL)-1) between the computations using aug-cc-pVTZ and aug-cc-pVQZ) suggesting that the basis set limit has been reached. The gas –phase computed values at B3LYP and CCSD are also shown in Table 1. Though these computations do get the correct sign of the rotation, they are appreciably off of the experimental values, and the percent error varies with wavelength. Cautionary point 2: it is not obvious what is the appropriate computational method to compute OR, and beware of values that seem reasonable at one wavelength – this does not predict a good agreement at a different wavelength.
Figure 1. Optimized geometry of 1 at B3LYP/aug-cc-pVTZ.
Lastly, computed solution values of the OR were performed with PCM and B3LYP. These values are given in Table 1. Again the agreement is poor. So cautionary point 3: computed (PCM) solution OR
may be in quite poor agreement with experiment.
Often the culprit to poor agreement between computed and experimental OR is attributed to omitted vibrational effects, but in this case, because 1 is so rigid, one might not expect too much error to be caused by the effects of vibrations. So the overall result – we need considerable work towards addressing how to accurately compute optical activity!
References
(1) Lahiri, P.; Wiberg, K. B.; Vaccaro, P. H.; Caricato, M.; Crawford, T. D. "Large Solvation Effect in the Optical Rotatory Dispersion of Norbornenone," Angew. Chem. Int. Ed. 2014, 53, 1386-1389, DOI: 10.1002/anie.201306339.
InChIs
1: InChI=1S/C7H8O/c8-7-4-5-1-2-6(7)3-5/h1-2,5-6H,3-4H2/t5-,6+/m1/s1
InChIKey=HUQXEIFQYCVOPD-RITPCOANSA-N

Henry Rzepa responded on 26 Feb 2014 at 12:47 am #
I think it interesting that more or less simultaneously with Bijvoet confirming that the Fischer representation of absolute configuration of sugars (a 50:50 guess) was correct using X-rays, Kirkwood had established the same result in 1951 by computing the optical rotation using quantum mechanics; 10.1063/1.1700491. IMHO, this chiroptical proof does not get the same publicity and credit that the anomalous X-ray scattering method does.
Perhaps of course Kirkwood was “lucky”, in selecting 2,3-epoxybutane for his calculations.
Another juxtaposition is that both Kirkwood and Bijvoet’s result came just in time to allow Watson and Crick’s DNA double helix to have the correct chirality (i.e. a right handed helix), otherwise that too might have been a 50:50 guess. Famously, Pauling’s helical model of peptides at around the same time was (initially) the wrong handedness, ie a left helix rather than a right helix (an even greater irony, since Kirkwood and Pauling worked in the same chemistry dept at the time!).
And when the time comes that every chemistry dept considers it essential to have a VCD-IR spectrometer in their instrumentation, the measurement and interpretation of optical rotations may fade away in importance.
Henry Rzepa responded on 26 Feb 2014 at 4:21 am #
Norbornenone is a classic pathological system (fortunately extreme examples such as this are rare). I offer here some results I just obtained using the new MN12L functional (which we have had great success with for other chiroptical properties).
MN12L/aug-cc-pVTZ/SCRF=cyclohexane; 633/1083, 589/1319, 355/9198° (see doi: 10042/27640). Oddly at the longer wavelengths it is no better than B3LYP, but at the shorter 355nm, it is almost spot on (of course it might be getting the correct answer for entirely the wrong reasons!). So the error is very non-linear. Which suggests that a deeper analysis/parametrisation of the DFT method might correct the longer wavelengths as well.
It is interesting that the vast majority of (old) polarimeters continue to measure just at 589nm, although the mercury line at 355nm has been available for a long time. The new digital breeds of polarimeters can go down to even shorter wavelengths.