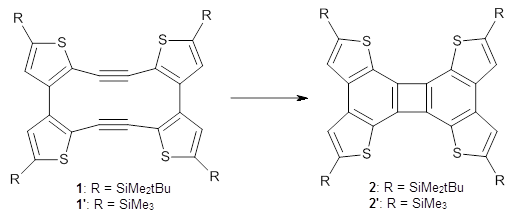
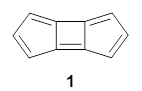
I was all set to write a review of an interesting study of bowtiene 1: its rearrangement to other C10H6 isomers and its dimerization. But as I was gathering my information, I wanted to prepare the images of the optimized geometries, and so I went to get the supplementary materials.
The author has a section on the supplementary materials that indicates it contains Cartesian coordinates – just what I need. (This section ends with the curious line “This material is available for free of charge via the internet at http://pubs.acs.org.”; it’s curious because the article is in a journal not published by ACS. I’ll leave for speculation just what happened here, but clearly the copy-editing done by the Canadian Journal of Chemistry is not quite up to snuff!)
So, I went to the website and clicked on the link for the supplementary material and was then told that I did not have access to this material and that either I had to become a subscriber or I had to purchase access to the article. (I should point out here that I received this article through interlibrary loan.) This is the first time that I have run into a paywall to get supporting materials! I know I am probably lucky that it took 7 years before running into this problem. But that makes this situation so frustrating – just why is the Canadian Journal of Chemistry placing supplementary material behind a paywall, especially when so few other publishers are doing this?
Well, until I get the supplementary materials, I will not write a post about this article.
New policy: I will not blog about an article unless (a) there is information on the 3-D structure of the molecules, typically in supporting materials, and (b) this information is available for free. This requirement should really be the minimum for publishing computational chemistry results. Now, I would also hope that the coordinates are readily reusable – see Henry Rzepa’s post about recent problems he’s run into!