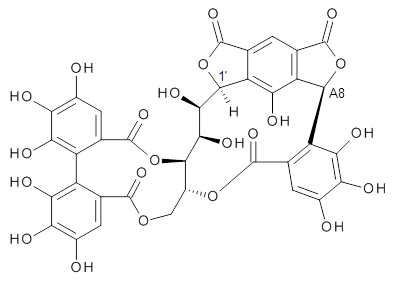
Here’s another nice example of the use of computed NMR spectra to aid in structure identification. Quercusnin A was identified in an extract of dried sapwood from the oak tree Quercus crispula. The NMR sectrum along with structural comparison to the previously determined extract vescalagin, led the authors to the structure 1.1
1
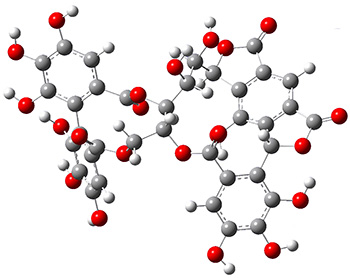
To aid in determining the absolute stereochemistry as centers 1’ and A8, the authors employed a computational approach. Conformers of the four diastereomers (RR, RS, SR, SS) were optimized first with molecular mechanics, then the low energy conformers were reoptimized at AM1, and then finally all of the conformers within 6 kcal mol-1 of the lowest energy structure were reoptimized at PCM(acetone)/B3LYP/6-31G(d,p). The 1H and 13C NMR chemical shifts for all of the structures that contribute greater than 1% to the Boltzmann population were computed at PCM(acetone)mPW1PW91/6-311+G(2d,p)//B3LYP/6-31G(d,p). The DP4 probability (see this post) identified the (1’S,A8R) isomer with 100% probability for matching up with the experimental NMR spectrum. Additionally, the computed ECD spectrum matches nicely with the experimental spectra for this same stereoisomer. The lowest energy conformer of 1 is shown in Figure 1.
|
1 |
Figure 1. PCM(acetone)/B3LYP/6-31G(d,p) structure of the lowest energy conformer of 1.
References
(1) Omar, M.; Matsuo, Y.; Maeda, H.; Saito, Y.; Tanaka, T. "New Metabolites of C-Glycosidic Ellagitannin from Japanese Oak Sapwood," Org. Lett. 2014, 16, 1378–1381, DOI: 10.1021/ol500146a.
InChIs
1: InChI=1S/C36H24O22/c37-11-2-8-15(24(44)20(11)40)16-9(3-12(38)21(41)25(16)45)36(53)56-29-14(5-54-32(8)49)55-33(50)10-4-13(39)22(42)26(46)19(10)30-17-6(34(51)57-30)1-7-18(23(17)43)31(58-35(7)52)28(48)27(29)47/h1-4,14,27-31,37-48H,5H2/t14-,27-,28-,29-,30-,31+/m1/s1
InChIKey=PTEZCLBSJBUWFI-LJBUUXJGSA-N