The Pascal group has synthesized dodecaphenyltetracene 1.1
While this paper has little computational work, it is of interest to readers of this blog since I have discussed many aspect of aromaticity. This new tetracene is notable for its large twisting along the tetracene axis: about 97° in the x-ray structure. I have optimized the structure of 1 at B3LYP-D3(BJ)/6-311G(d) and its structure is shown in Figure 1. It is twisted by about 94°. The computed and x-ray structures are quite similar, as seen in Figure 2. Here the x-ray structure is shown with red balls, the computed structure with gray balls, and hydrogens have been removed for clarity.
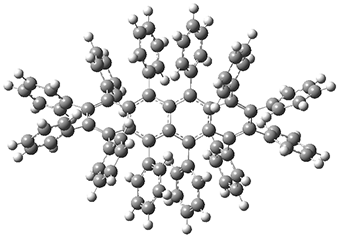
Figure 1. B3LYP-D3(BJ)/6-311G(d) optimized structure of 1.
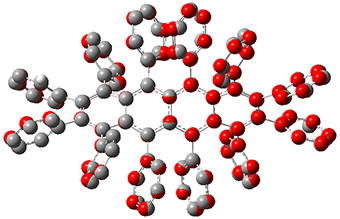
Figure 2. Comparison of the x-ray (red) and computed (gray) structures of 1. (Hydrogens omitted for clarity.)
The authors note that this molecule is chiral, having near D2 symmetry. (The optimized structure has D2 symmetry.) They performed AM1 computations to estimate a very low barrier for racemization of only 17.3 kcal mol-1, leading to a half-life of less than one second at RT.
A notable aspect of the molecule is that aromaticity can adapt to significant twisting yet retain aromatic character. For example, the molecule is stable even surviving boiling off of chloroform (61 °C) to form crystals and the majority of the C-C bonds in the tetracene portion have distances typical of aromatic systems (~1.4 Å).
References
1) Xiao, Y.; Mague, J. T.; Schmehl, R. H.; Haque, F. M.; Pascal Jr., R. A., “Dodecaphenyltetracene.” Angew. Chem. Int. Ed. 2019, 58, 2831-2833, DOI: 10.1002/anie.201812418.
InChIs
1: InChI=1S/C90H60/c1-13-37-61(38-14-1)73-74(62-39-15-2-16-40-62)78(66-47-23-6-24-48-66)86-82(70-55-31-10-32-56-70)90-84(72-59-35-12-36-60-72)88-80(68-51-27-8-28-52-68)76(64-43-19-4-20-44-64)75(63-41-17-3-18-42-63)79(67-49-25-7-26-50-67)87(88)83(71-57-33-11-34-58-71)89(90)81(69-53-29-9-30-54-69)85(86)77(73)65-45-21-5-22-46-65/h1-60H
InChIKey=NJQABVWYMCSFNE-UHFFFAOYSA-N
