A significant portion of Chapter 4 of my book is devoted to phenylnitrene 2 and phenylcarbene. Phenyloxenium cation 1 is isoelectronic with phenylnitrene and so one might expect similar behavior of the two. Winter has reported a nice computational study of the singlet and triplet phenyloxenium cation and finds some very striking differences between phenyloxenium cation and phenylnitrene.1
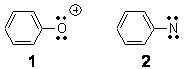
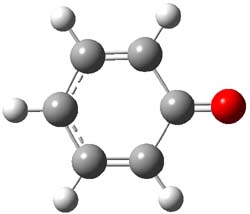
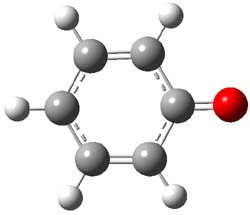
Phenylnitrene has a triplet ground state, with the 1A1 state about 18 kcal mol-1 higher in energy, and the 1A2 state higher still. CASPT2/pVTZ//CASSCF(8,8)/pVTZ computations of 1 find the singlet 1A1 to be the ground state. The lowest triplet is 22.1 kcal mol-1 higher in energy, and the lowest 1A1 state lies 30.8 kcal mol-1 above the ground state singlet. (The structures of the lowest singlet and triplet of 1 are shown in Figure 1.) Reanalysis of the ultraviolet photoelectron spectrum of the phenoxy radical2 switches the assignments of the observed transitions and is in excellent agreement with these computed values. G3 and CCSD(T)/cc-pVTZ predicts a similar value for the singlet-triplet gap. B3LYP, MPW1PW91, and some other DFT methods predict the singlet to be lower in energy than the triplet, but with a gap half of the correct value of 22 kcal mol-1.
|
1 singlet (1A1) |
1 triplet (3A2) |
Figure 1. CASSCF(8,8) optimized geometries of the lowest singlet and triplet states of 1.
The origin of the difference between 1 and 2 lies in the description of the singlet state. The singlet state of 1 places the two lone pairs on oxygen into the sp-like orbital and into the in plane p orbital. However, in 2, the singlet is described by two determinants, one with the nitrogen lone pairs in the sp and in plane p orbital and the second determinant has them in the sp orbital and in the perpendicular p orbital. For 1, this single determinant allows for the positive charge to delocalize into the phenyl ring and off the very electronegative oxygen; this is manifest in a short C-O bond (1.211 Å). The greater electronegativity of oxygen then nitrogen brings the perpendicular p orbital lower in energy and better able to mix with the phenyl π-orbitals. In other words, the greater electronegativity of O over N results in a large symmetry break of the degenerate p orbitals.
References
(1) Hanway, P. J.; Winter, A. H., "Phenyloxenium Ions: More Like Phenylnitrenium Ions than Isoelectronic Phenylnitrenes?," J. Am. Chem. Soc., 2011, 133, 5086-5093, DOI: 10.1021/ja1114612
(2) Dewar, M. J. S.; David, D. E., "Ultraviolet photoelectron spectrum of the phenoxy radical," J. Am. Chem. Soc., 1980, 102, 7387-7389, DOI: 10.1021/ja00544a050