Benzene is certainly one of the most iconic chemical compounds – its planar hexagonal structure is represented often in popular images involving chemists, and its alternating single and double bonds the source of one of chemistry’s most mythic stories: Kekule’s dream of a snake biting its own tail. So while the structure of benzene is well-worn territory, what of the structure of the benzene dication? Jasik, Gerlich and Rithova probe that question using a combined experimental and computational approach.1
The experiment involves generation of the benzene dication at low temperature and complexed
to helium. Then, using infrared predissociation spectroscopy (IRPD), they obtained a spectrum that suggested two different structures.
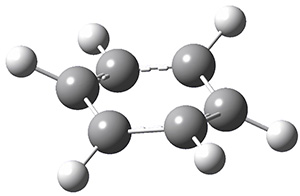
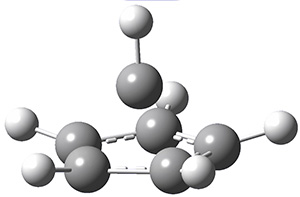
Next, employing MP2/aug-cc-pVTZ computations, they identified a number of possible geometries, and the two lowest energy singlet dications have the geometries shown in Figure 1. The first structure (1) has a six member ring, but the molecule is no longer planar. Lying a bit lower in energy is 2, having a pentagonal pyramid form. The combination of the computed IR spectra of each of these two structures matches up extremely well with the experimental spectrum.
|
1 |
2 |
Figure 1. MP2/aug-cc-pVTZ geometries of benzene dication 1 and 2.
References
(1) Jašík, J.; Gerlich, D.; Roithová, J. "Probing Isomers of the Benzene Dication in a Low-Temperature Trap," J. Am. Chem. Soc. 2014, 136, 2960-2962, DOI: 10.1021/ja412109h.

Henry Rzepa responded on 09 Apr 2014 at 9:40 am #
I recognise 2! It was suggested by myself in 10.1038/nchem.596 as part of the rational design of a bond to He! Species 2 is also isoelectronic to a BBr(+) cation (i.e. CH(2+)), for which a crystal structure is reported (doi: 10.1016/0022-328X(94)05089-T.
See here.
When one moots such weird and wacky species, it is not often in the expectation that someone will identify them a few years later in unrelated work.
Henry Rzepa responded on 10 Apr 2014 at 4:19 am #
I am a little intrigued regarding the reported energy difference between 1 and 2, reportedly 60 kJ/mol in favour of the latter. That is rather more than a “bit more”? If the two species are in equilibrium, the concentration of the former would be far to low to be observed. Perhaps it is a kinetic product, and the barrier to conversion to 2 prevents this. But in which case, how is 2 formed in the first place?
A connected world (journals and blogs): The benzene dication. « Henry Rzepa's blog responded on 08 Jul 2015 at 10:59 am #
[…] rested there until yesterday, when I spotted this on Steve’s blog where he discusses this recent article on the structure of the benzene […]