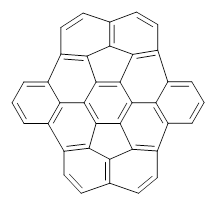
I have discussed a few bowl-shaped aromatics in this blog (see for example this and this). Kuo and Wu now report on a few bowls derived from C70-fullerenes.1 The bowl 1 was synthesized (along with a couple of other derivatives) and its x-ray structure obtained. As anticipated this polyclic aromatic is not planar, but rather a definite bowl, with a bowl depth of 2.28 Å. This is less curved than when the fragment is present in C70-fullerene.
|
|
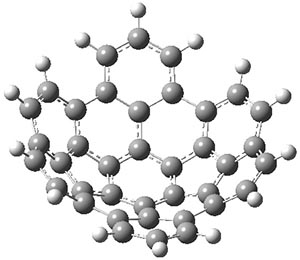
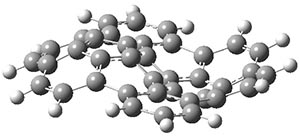
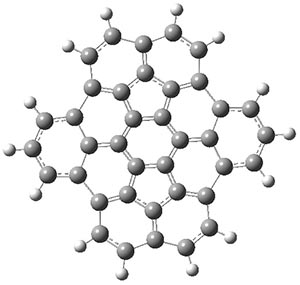
Interestingly, this bowl does not invert through a planar transition state. The fully planar structure 1pl, shown in Figure 1, is 116 kcal mol-1 above the ground state bowl structure, computed at B3LYP/cc-pVDZ. Rather, the molecule inverts through a twisted S-shaped structure 1TS, also shown in Figure 1. The activation barrier through 1TS is 80 kcal mol-1. This suggests that 1 is static at room temperature, unlike corranulene which has an inversion barrier, through a planar transition state, of only 11 kcal mol-1. The much more concave structure of 1 than corranulene leads to the greatly increased strain in its all-planar TS. This implies that properly substituted analogues of 1 will be chiral and configurationally stable. Not remarked upon is that the inversion pathway, which will interchange enantiomers when 1 is properly substituted, follows a fully chiral path, as discussed in this post.
|
1 |
|
|
1TS |
1pl |
Figure 1. B3LYP/cc-pVDZ optimized geometries of 1, 1TS, and 1pl.
Reference
(1) Wu, T.-C.; Chen, M.-K.; Lee, Y.-W.; Kuo, M.-Y.; Wu, Y.-T. "Bowl-Shaped Fragments of C70 or Higher Fullerenes: Synthesis, Structural Analysis, and Inversion Dynamics," Angew. Chem. Int. Ed. 2013, 52, 1289-1293, DOI: 10.1002/anie.201208200.
InChIs
1: InChI=1S/C38H14/c1-3-17-21-11-7-15-9-13-23-19-5-2-6-20-24-14-10-16-8-12-22-18(4-1)27(17)33-35-29(21)25(15)31(23)37(35)34(28(19)20)38-32(24)26(16)30(22)36(33)38/h1-14H
InChIKey=KLCLRPVBVWXTPN-UHFFFAOYSA-N