In 1955 Mislow1 discussed the possibility of enatiomers interchanging via a path that was entirely chiral, never passing through an achiral structure. His analogy is the inversion of a rubber glove, taking a right hand rubber glove and pulling it inside out creates a left hand glove (its mirror image) but never passing through an achiral glove. Well, now a helicene with this type of stereochemistry has been developed, with a stable chiral intermediate.2
Helicenes typically interchange (P → M → P) through an achiral saddle-like structure. But larger helicenes can have high-lying intermediates along this pathway. Helquat P-1 interchanges to M-1 through the intermediate 2, which is an achiral structure and can be isolated.

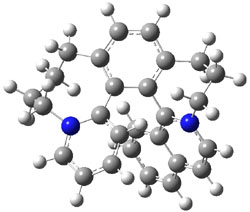
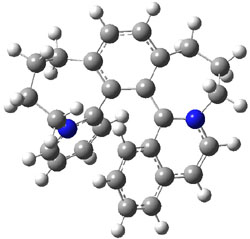
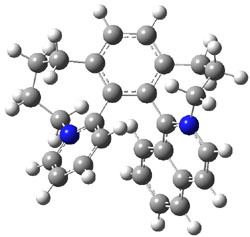
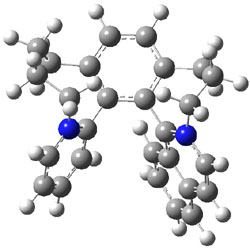
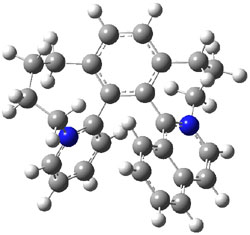
Computations at B3LYP/def2-TZVP//PBE/def2-SV(P) with dispersion corrections (and PCM simulating DMSO) of the inversion process identified a number of intermediates and transition states along the stereoinversion pathway. The intermediate 2 lies 18.4 kJ mol-1 above 1. These structures are shown in Figure 1. The highest lying TS between 2 and P-1 (labeled TSP in Figure 1) is 119 kJ mol-1 above 2. The highest lying TS on the path from 2 to M-1 (labeled TSM in Figure 1) is 138 kJ mol-1 above 2. Note that going from 2 to P-1 is not the mirror image path of going from 2 to M-1.
|
TSP |
TSM |
|
P-1 |
2 |
M-1 |
Figure 1. Optimized structures of 1, 2, and the highest transition states interconverting them.
Heating racemic 2 and following the conversion to 1 with NMR gives the activation barrier of 119 kJ mol-1, in excellent agreement with the computation.
Racemic 2 was resolved through differential crystallization and its x-ray structure indicates it is (+)-[Sa,Ra]. Heating it does give just P-1, as predicted by the computations. Then heating P-1 to 180 °C does racemize it, with an experimental barrier of 157.7 kJ mol-1. The computations predict a barrier of 156.6 kJ mol-1, again in fine agreement with experiment. Overall, a nice piece showing experiment and computation working together to provide an understanding of an interesting chemical system!
References
(1) Mislow, K.; Bolstad, R., "Molecular Dissymmetry and Optical Inactivity," J. Am. Chem. Soc., 1955, 77, 6712-6713, DOI: 10.1021/ja01629a131
(2) Adriaenssens, L.; Severa, L.; Koval, D.; Cisarova, I.; Belmonte, M. M.; Escudero-Adan, E. C.; Novotna, P.; Sazelova, P.; Vavra, J.; Pohl, R.; Saman, D.; Urbanova, M.; Kasicka, V.; Teply, F., "[6]Saddlequat: a [6]helquat captured on its racemization pathway," Chem. Sci. 2011, ASAP, DOI: 10.1039/C1SC00468A
InChIs
1: InChI=1/C27H26N2/c1-2-11-23-20(8-1)15-19-29-18-7-10-22-14-13-21-9-3-5-16-28-17-6-4-12-24(28)25(21)26(22)27(23)29/h1-2,4,6,8,11-15,17,19H,3,5,7,9-10,16,18H2/q+2
InChIKey=YVVUWUZXUZDMQS-UHFFFAOYAP

Henry Rzepa responded on 25 Oct 2011 at 6:06 am #
Chirally continuous pathways are indeed intriguing. One deceptively simple system which has some of these features is in fact the axial/equatorial substituent flipping of cyclohexane. On Bob Hanson’s page, you will find one possible potential energy pathway for it do do this. This shows a symmetrical potential, with a twist boat minimum at the half way stage. This, and the preceding half-chair both have C2 chiral symmetry. Thus in this representation, most of the potential involves a chiral species, and only the start and end chairs are achiral. Another, possibly more common, representation of this ring flip involves a boat transition state (of C2v symmetry) at the half way point. This represents the more conventional mechanism for enantiomerisations, in which the mid point (transition state) has a plane of symmetry which allows reflection of one enantiomer on to the other and hence is not chirally continuous.
Michael Mauksch responded on 07 Nov 2011 at 10:32 am #
Another factor to consider for these Euclidean (as opposed to topological) rubber glove molecules as in the example above is the number of atoms involved. In a multi-atom molecule it is less difficult to realize a non-synchronous movement of two reaction coordinate components, to ensure a completely chiral enantiomerization pathway, e.g. as in a o,o’-ethyl bridged biphenyl of Müllen et al.
Mezey had proven set theoretically that to enantiomerize via a completely chiral pathway, a chiral molecule should have at least five atoms. For a demonstrative example see: ACIEE 1997, 36, 1856.
DOI: 10.1002/anie.199718561