Synthetic application of the Bergman cyclization is rare. Basak reports a real interesting use of this reaction to create polycyclic aromatics.1 So, for example, heating up 1 in DMSO leads to the 4helicene 2. The proposed mechanism is shown in Figure 1. The Bergman cyclization leads to the biradical 3, which adds to the pendant phenyl group to give 4. Hydrogen abstraction then gives 5, which abstracts hydrogens from the solvent to produce 6. (Use of DMSO-d 6 provides deuterium incorporated products consistent with the diradical shown in 4.) Oxidation then gives the final product 2.

Figure 1. Proposed mechanism for the conversion of 1 to 2.
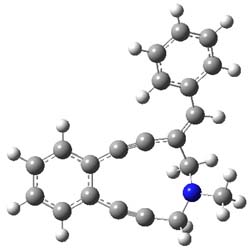
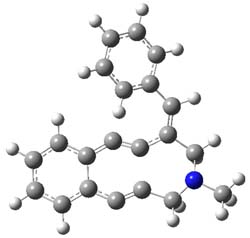
B3LYP computations were performed to examine the relative rates with substituents on the phenyl ring. The structure of 1’ (with a methyl group replacing the Ns group – 4-nitrobenzenesulfonyl) and the transition state for the Bergman cyclization are shown in Figure 2. Unfortunately, computations were not used to analyze the complete proposed mechanism – a project that awaits the eager student perhaps?
|
1’ |
1’TS |
Figure 2. B3LYP/def2-TZVP//BP86/def2-TZVP optimized structures of 1’ and transition state for the Bergman cyclization of 1’.
References
(1) Roy, S.; Anoop, A.; Biradha, K.; Basak, A., "Synthesis of Angularly Fused Aromatic Compounds from Alkenyl Enediynes by a Tandem Radical Cyclization Process," Angew. Chem. Int. Ed., 2011, 50, 8316-8319, DOI: 10.1002/anie.201103318
InChIs
1’: InChI=1/C21H17N/c1-22-15-7-12-20-10-5-6-11-21(20)14-13-19(17-22)16-18-8-3-2-4-9-18/h2-6,8-11,16H,15,17H2,1H3/b19-16+
InChIKey=MKFCCTRNSOHUDU-KNTRCKAVBS
2’: InChI=1/C21H17N/c1-22-12-16-10-14-6-2-4-8-18(14)21-19-9-5-3-7-15(19)11-17(13-22)20(16)21/h2-11H,12-13H2,1H3
InChIKey=ZNCMIYYKBITIMW-UHFFFAOYAR