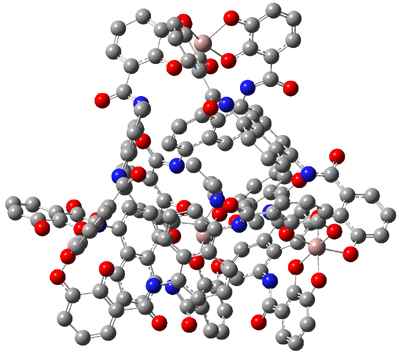
Bergman and Raymond have prepared a Ga4L612- host that can encapsulate small monocations and neutral species.1,2 Figure 1 shows the host with an encapsulated tetraethylammonium ion NEt4+. (Note that the hydrogens have been suppressed for easier viewing. And be sure to click on the structure in order to interact with the 3-D model.)
Figure 1. Structure of the Ga4L612- host encapsulating NEt4+.
Of interest for readers of this blog is that they have now computed the NMR spectra of the encapsulated species.3 The geometry of the host is fixed to that found in the crystal structure where Cp*Co is the guest and the geometry of the guest (NEt4+, PEt4+ and others) is optimized with molecular mechanics. The complex is then computed at B3LYP with the 3-21G basis set for the host and the G-311(g,p) basis set for the guest. The computed 1H chemical shifts are actually within 0.1 ppm of experiment, and show the swapping of the relative position of the chemical shifts of the methyl vs methylene proton for the two guests.
This demonstrates the computed NMR shifts can be applied to some very large molecules including organometallics.
References
(1) Caulder, D. L.; Powers, R. E.; Parac, T. N.; Raymond, K. N., "The Self-Assembly of a Predesigned Tetrahedral M4L6 Supramolecular Cluster," Angew. Chem. Int. Ed. 1998, 37, 1840-1843, DOI: 10.1002/(SICI)1521-3773(19980803)37:13/14<1840::AID-ANIE1840>3.0.CO;2-D
(2) Biros, S. M.; Bergman, R. G.; Raymond, K. N., "The Hydrophobic Effect Drives the Recognition of Hydrocarbons by an Anionic Metal-Ligand Cluster," J. Am. Chem. Soc. 2007, 129, 12094-12095, DOI: 10.1021/ja075236i
(3) Mugridge, J. S.; Bergman, R. G.; Raymond, K. N., "1H NMR Chemical Shift Calculations as a Probe of Supramolecular Host-Guest Geometry," J. Am. Chem. Soc. 2011, 133, 11205-11212, DOI: http://dx.doi.org/10.1021/ja202254x