Since Heilbronner1 proposed the Möbius annulene in 1964, organic chemists have been fascinated with this structure and many have tried to synthesize an example. I have written many blog posts (1, 2, 3, 4, 5) related to computed Möbius compounds. Now, Herges and Grimme and co-workers have looked at cationic Möbius annulenes.
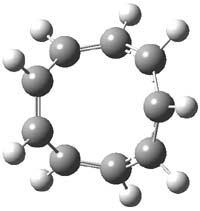
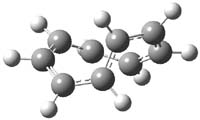
For the [9]annulene cation,2 a variety of DFT methods, along with SCS-MP2 and CCSSD(T) computations suggest that the lowest energy Hückel (1h) and Möbius (1m) structures, shown in Figure 1, are very close in energy. In fact, the best estimate (CCSD(T)/CBS) is that they differ by only 0.04 kcal mol-1. Laser flash photolysis of 9-chlorobicyclo[6.1.0]nona-2,4,6-triene suggest however that only the Hückel structure is formed, and that its short lifetime is due to rapid electrocyclic ring closure.
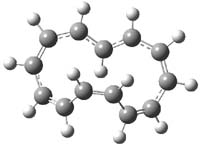
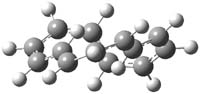
In a follow-up study, Herges has examined the larger annulene cations, specifically [13]-, [17]- and [21]-annulenes. 3 The Möbius form of [13]-annulene cation (2m) is predicted to be 11.0 kcal mol-1 lower in energy that the Hückel (2h) form at B3LYP/6-311+G**. The structures of these two cations are shown in Figure 1. The Möbius cation 2m is likely aromatic, having NICS(0)= -8.95. Electrocyclic ring closure of 2m requires passing through a barrier of at least 20 kcal mol-1, suggesting that 2m is a realistic target for preparation and characterization.
|
1h |
1m |
|
2h |
2m |
Figure 1. Optimized structures of 1 (CCSD(T)/cc-pVTZ)2 and 2 (B3LYP/6-311+G**)3.
The energy difference between the Möbius and Hückel structures of the larger annulenes is very dependent on computational method, but in all cases the difference is small. Thus, Herges concludes that [13]-annulene cation should be the sole target of synthetic effort toward identification of a Möbius annulene. Experimental studies are eagerly awaited!
References
(1) Heilbronner, E., “Huckel molecular orbitals of Mobius-type conformations of annulenes,” Tetrahedron Lett., 1964, 5, 1923-1928, DOI: 10.1016/S0040-4039(01)89474-0.
2) Bucher, G.; Grimme, S.; Huenerbein, R.; Auer, A. A.; Mucke, E.; Köhler, F.; Siegwarth, J.; Herges, R., "Is the [9]Annulene Cation a Möbius Annulene?," Angew. Chem. Int. Ed., 2009, 48, 9971-9974, DOI: http://dx.doi.org/10.1002/anie.200900886
(3) Mucke, E.-K.; Kohler, F.; Herges, R., "The [13]Annulene Cation Is a Stable Mobius Annulene Cation," Org. Lett., 2010, 12, 1708–1711, DOI: 10.1021/ol1002384
InChIs
1: InChI=1/C9H9/c1-2-4-6-8-9-7-5-3-1/h1-9H/q+1/b2-1-,5-3-,6-4-,9-7-
InChIKey=LIUDWUIEJKKGNI-BWYSQNKRBF
2: InChI=1/C13H13/c1-2-4-6-8-10-12-13-11-9-7-5-3-1/h1-13H/q+1/b2-1-,5-3-,6-4-,9-7-,10-8-,13-11-
InChIKey=FUBPZYTZTJGXKZ-OGBOFXOGBR

Henry Rzepa responded on 11 Jun 2010 at 1:27 am #
One property identified for Mobius bands is writhe, which arises from deviation from a pure plane of the so-called centre line of the band. The more the band deviates into three dimensions, the greater the writhe, and the less the twist. The writhe is important because of a theorem which states that the sum of the writhe and the remaining twist is an integer (in units of π), such that
Lk =Tw + Wr (see DOI: 10.1021/ja710438j).
The linking number Lk for a formal Mobius band is 1π. An assumption, as yet untested experimentally, is that writhe can reduce the twist, and hence the loss of overlap in adjacent pπ-pπ interactions in a conjugated annulene. This might in theory result in greater stability. So I suggest that for every putative Möbius system which is proposed for synthesis, its writhe be reported. For 2m discussed above, the values are:
Lk = 1, Tw = 0.646, Wr = 0.353
I note that for the hypothetical trefoil knot I discussed on my blog, for which Lk =6, the writhe had the value of 6.8π. This merely proves that it is actually possible to contrive twisted molecules where most of the twist is actually writhe!
Computational Organic Chemistry » [36]-annulene responded on 01 Nov 2010 at 9:09 am #
[…] can twist, and I have blogged about a number of examples (cations and neutrals). The twist can be portioned into a twist associated with the dihedral angle as one […]
Michael Mauksch responded on 09 Feb 2011 at 8:37 am #
I have written in chapter 5 of my 1999 doctoral thesis with Paul Schleyer:
“The aromatic charged annulenes in the Möbius topology, (CH)9+, (CH)11- and
(CH)13+, are all more stable than any other 4n electron annulene conformation we have been able to locate on their respective potential surfaces.”
For me, it only comes at a surprise that it took that long before that prediction was (basically) confirmed, if only computationally ..
Michael Mauksch responded on 10 Feb 2011 at 1:02 am #
..for easy reference I am giving the following link to my thesis:
http://deposit.ddb.de/cgi-bin/dokserv?idn=985500352
Michael Mauksch responded on 28 Sep 2012 at 1:25 am #
Interestingly, I found out lately that due to purported license issues, the German National Library has removed my doctoral thesis from their internet server (while others are still available ..). So if you’d still like to access the book free of charge, you’d need to follow only the “official” link now to the Erlangen university document server:
http://www.opus.ub.uni-erlangen.de/opus/volltexte/2007/679/