I concluded the subchapter on pseudopericyclic reaction (Chapter 3.4) with a discussion of the controversy concerning the nature of the electrocyclization of 7-azahepta-1,2,4,6-tetraene 1. Quickly summarizing form the book, based on which data one deems important, the reaction can be seen as either pericyclic or pseudopericyclic.
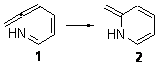
However, I offered David Birney’s opinion as perhaps the proper way to interpret this reaction. David suggested that the TS has both pericyclic and pseudopericyclic character. I wrote that “what we have is a continuum from pericyclic to pseudopericyclic character, analogous to the SN1 to SN2 continuum for nucleophilic substitution”.
Duncan has revisited this reaction,1 employing both B3LYP and CASSCF(10,9) computations. He concludes that the reaction is “neither purely pericyclic nor pseudopericyclic” – just as Birney had indicated. Duncan does offer the possibility of a secondary orbital interaction involving the nitrogen lone pair. But it is nice to see confirmation of the interpretation that David originated for my book!
References
(1) Duncan, J. A.; Calkins, D. E. G.; Chavarha, M., "Secondary Orbital Effect in the Electrocyclic Ring Closure of 7-Azahepta-1,2,4,6-tetraene – A CASSCF Molecular Orbital Study,", J. Am. Chem. Soc., 2008, 130, 6740-6748, DOI: 10.1021/ja074402j.
InChIs
1: InChI=1/C6H7N/c1-2-3-4-5-6-7/h3-7H,1H2/b5-4-,7-6+
InChIKey=VCEMZTODUGWAPF-SCFJQAPRBS
2: InChI=1/C6H7N/c1-6-4-2-3-5-7-6/h2-5,7H,1H2
InChIKey=JGSLKNWXPRDWBA-UHFFFAOYAH

Computational Organic Chemistry » Pseudopericyclic [3,3]-sigmatropic Rearrangement responded on 08 Jun 2010 at 7:51 am #
[…] rearrangement, 1 and what is particularly interesting is how rare this seems to be! (See this post for an earlier related study.) Using CASSCF/6-31G* computations of Reactions 1-9, only Reaction 1 […]