One of the great longstanding dreams of synthetic and theoretical organic chemists is to prepare a stable molecule containing a pentacoordinate carbon atom. Bickelhaupt and co-workers propose a novel series of compounds that hint that this might be possible.1
Their attack is to first find a CR3 radical that is stable in its planar form. The nitrile group perfectly satisfies this goal. Next they look at the series of compounds X-C(CN)3-X– (1) where X is a halogen, searching for a stable D3h structure. This is found with the halogens: Br, I, and At, at the ZORA-OLYP/TZ2P level. Seems like case closed, except that inspection of the supporting materials shows that the nature of the D3h structure is sensitive to computational method. So, with the larger basis set ZORA-OLYP/QZ4P or with ZORA-OPBE/TZ2P, only the I and At compounds are local D3h minima. And with ZORA-M06/TZ2P, only the At compound is a local minimum. The authors do mention these points at the end of the article. So, what we have here is a tantalizing suggestion for how to prepare a hypercoordinate carbon species, but further computational (and experimental) work is clearly needed.
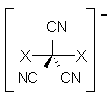
1: X = F, Cl, Br, I, At
References
(1) Pierrefixe, S. C. A. H.; van Stralen, S. J. M.; van Strale, J. N. P.; Guerra, C. F.; Bickelhaupt, F. M., "Hypervalent Carbon Atom: "Freezing" the SN2 Transition State," Angew. Chem. Int. Ed., 2009, 48, 6469-6471, DOI: 10.1002/anie.200902125
InChIs
1(F): InChI=1/C4F2N3/c5-4(6,1-7,2-8)3-9/q-1, InChIKey=LBDHZXPWKBFYBC-UHFFFAOYAX
1(Cl): InChI=1/C4Cl2N3/c5-4(6,1-7,2-8)3-9/q-1, InChIKey=NMFGEVWEEWBFSS-UHFFFAOYAU
1(Br): InChI=1/C4Br2N3/c5-4(6,1-7,2-8)3-9/q-1, InChIKey=FHKJQJBDHAEQES-UHFFFAOYAC
1(I): InChI=1/C4I2N3/c5-4(6,1-7,2-8)3-9/q-1, InChIKey=FWKBAUUEXUSUNH-UHFFFAOYAO
1(At): InChI=1/C4At2N3/c5-4(6,1-7,2-8)3-9/q-1, InChIKey=BJQBFKCUAROAAS-UHFFFAOYAK

Eutactic responded on 15 Sep 2009 at 10:42 pm #
Hi,
Certainly a fascinating read. However, I’m fairly certain (based on the paper and the ADF 2008.01 spec) that ‘ZORA-OLYP/TZ4P’ should actually be ‘QZ4P’.
Steven Bachrach responded on 16 Sep 2009 at 8:16 am #
Thanks for the correction – I have modified the post accordingly.
Henry Rzepa responded on 17 Sep 2009 at 9:56 am #
Another interesting point is that in the pantheon of normality, it is carbon that may be abnormal and silicon that is in fact normal. Thus what is surprising is not that a pentacoordinate carbon might exist, but why it is that the SN2 reaction actually does have a barrier!
I venture to suggest that the difficulty in forming a stable pentacoordinate carbon and the reason why organofluorine does not form strong hydrogen bonds (unlike eg organo-oxygen or organonitrogen) have at their core the same reason, ie the smallness of carbon (in the first example) and the smallness of fluorine (in the second).
As it happens, carbon could be regarded as abnormal in many other ways. We have just submitted an article in which, in effect, we argue that another icon of normality, benzene, is in fact quite abnormal!
Steven Bachrach responded on 17 Sep 2009 at 12:16 pm #
Actually, our work on SN2 reactions at sulfur and selenium and phosphorus have led us to a similar conclusion – that perhaps carbon (and nitrogen and oxygen) are the outliers on the periodic table. We find that with these larger atoms, substitution takes place in an addition-elimination fachion. And like you suggest, we believe that carbon is simply too small to accommodate 5 “ligands”.
This may be the tyranny of the organic chemists – to think that the world revolves around a “normal” carbon atom.
Henry Rzepa responded on 18 Sep 2009 at 1:02 am #
Responding to Steve, it could also be argued that the concept of valency arose out of the study of carbon (and nitrogen/oxygen), on the assumption that these represented normality. Notice for example the very careful use of the word pentacoordinate rather than pentavalent in the comments above. If one were to start from the premise that pentacoordinate is in fact normal, then the simple rules of valency would start having a rather difficult time. In fact pentacoordinate carbon is NOT pentavalent (in the sense of the two-electron Lewis covalent bond), and its valence shell is NOT expanded to ten. Rather, each of the bonds in such a species is defined by less than two electrons, and we loose the simple two electron Lewis picture (i.e. when we draw a line representing a bond, and then push curly arrows to represent mechanism, we no longer push integer units of electrons) and much of the pedagogic simplicity of introductory chemistry becomes complicated.
This becomes only too apparent in contemporary silicon chemistry, where the current vocabulary for describing bonding really can start to suffer. Topological analysis of the bonding all to frequently produces bonds defined by conspicuously non-integer numbers of electrons. So in that sense, we should be very grateful that most (abnormal) carbon chemistry was discovered before (normal) silicon chemistry.
I did write a short article with the delightful title The importance of being bonded (I can describe it as such, since the title was coined not by myself but by the journal editor!) which covers some of these themes. Its due out October, and perhaps when the DOI is available, I could ask Steve to edit it into this post? I presume an American audience is familiar with Oscar Wilde? Oh, Steve will like the exploratorium associated with this article (he has posted frequently on the importance of having data).
Henry Rzepa responded on 22 Sep 2009 at 1:05 am #
The recent experimental characterisation of a stable hexacoordinate carbon should be added to this discussion of stable hypercoordinate carbon species (DOI: 10.1021/ja710423d), lest the impression is given that such species are still a dream rather than reality. Here we have an accurate crystal structure, and furthermore, the measured electron density has been subjected by the authors to a topological AIM analysis, which shows bond critical points in the relevant region. If you read the article quoted further, you will notice these authors thank a (hitherto anonymous) referee (Ref 12) for pointing out that such hypercoordinate interactions are actually very common in the Cambridge crystal structure database. In fact, the Sn2 transition state discussed in this blog post, and the double Sn2-like structure of the hexacoordinate carbon noted above are not that fundamentally different. One way of regarding the structure reported in 10.1021/ja710423d is of a divalent dication, coordinated further by two pairs of orthogonal O…C…O interactions, each of which is analogous to the hypothetical X(-)…C(+)…X(-) interaction reported by Bickelhaupt et al.
In 10.1021/ja710423d, a tentative suggestion is made that hypervalency is being exhibited. In fact, this must be very contentious. Although bond critical points do exist in the AIM analysis, if instead an ELF analysis is performed, I venture to suggest one will not find disynaptic basins containing any significant population of electrons in the C…O regions. The interaction is indeed almost purely ionic, and I would contend, hypervalency is NOT exhibited. Bickelhaupt et al of course in their own article claim this in its title, but the analogy to 0.1021/ja710423d I would suggest would lead to the conclusion that the bonding is ionic, and no hypervalency is being exhibited (in other words, an ELF integration would show that the total number of electrons in synaptic basins associated with the central carbon would not exceed eight).
Various other authors have of course also put forward many a diverse suggestion for hyper-coordination of carbon; one only has to do a CAS search to reveal this!
Steven Bachrach responded on 22 Sep 2009 at 7:35 am #
I have blogged on the paper Henry refers to “Synthesis and Structure of a Hexacoordinate Carbon Compound” – see my blog post
Henry Rzepa responded on 22 Sep 2009 at 9:31 am #
Determined to mix things up, I have continued the discussion of this theme on my own blog (http://www.ch.imperial.ac.uk/rzepa/blog/?p=783), where I propose a brand new candidate for a frozen SN2 transition state, and one which has five C…C “bonds” surrounding the central atom, making it mainstream organic chemistry.
I suppose the interesting issue is whether I should have done so on a blog! Should I instead have saved it up for a (probably protracted) submission to a more conventional scientific forum? At any rate, if you want to find out what my suggestion is, visit my blog! (Sorry Steve, your blog has quite enough hits already, and I am jealous of them:-)
Henry Rzepa responded on 24 Sep 2009 at 1:10 am #
I noted in an earlier post that Nature Chemistry would be publishing a commentary on The importance of being bonded. Its now out at 10.1038/nchem.373, and includes an interactive figure where various bonding features can be explored (accessible directly here). I include this direct link because Nature Chemistry have an interesting publication policy, which Steve might be interested in. The article itself is available for one month for free, after which one has to have a subscription to the journal. The interactive figure (being largely data) will remain free for ever, but thereafter the link to it will only be available via the journal pages from the (now non free) article. However, I am allowed to expose that link elsewhere (as here for example) so that it continues to be accessible after one month for anyone without a subscription to the journal.
Oh, by the way, I notice that astatine (X in the frozen SN2 transition state) only exists as a few atoms at a time. It would be reasonable to conclude that no-one is likely to make gram quantities of the proposed compound any time soon!
Yan-Bo Wu responded on 08 Apr 2010 at 9:32 pm #
Hi, everyone
I have no ADF package, so I can not check such SN2 structure with the methods used in the 10.1002/anie.200902125 paper.
I studied the At-C(CN)3-At (-) with B3LYP/aug-cc-pVTZ (aug-cc-pVTZ-PP for At) using Gaussian03. Though it is an energy minimum, its wavefunction is instable (see below). So we need further verification.
G03 results
Eigenvectors of the stability matrix:
Eigenvector 1: Triplet-A2″ Eigenvalue=-0.0036675
44 -> 49 0.70504
Eigenvector 2: Triplet-E’ Eigenvalue= 0.0213356
48 -> 49 0.70512
Eigenvector 3: Triplet-E’ Eigenvalue= 0.0213356
47 -> 49 0.70512
Eigenvector 4: Triplet-E” Eigenvalue= 0.0242906
46 -> 49 0.70591
Eigenvector 5: Triplet-E” Eigenvalue= 0.0242909
45 -> 49 0.70591
Eigenvector 6: Triplet-A1′ Eigenvalue= 0.0352397
34 -> 49 0.10460
43 -> 49 0.69103
The wavefunction has an RHF -> UHF instability.
Computational Organic Chemistry » Helium Bonds responded on 22 Jun 2010 at 12:54 pm #
[…] study of potential stable molecules containing a bond to helium.1 The work was inspired by the post on this blog pertaining to potential hypervalent carbon species that mimic the SN2 transition s…. Rzepa first reported some of his results on his own blog (see this post and previous ones). The […]
Capturing penta-coordinate carbon! (Part 1). « Henry Rzepa responded on 03 Apr 2011 at 12:20 am #
[…] to be said. In the manner of the blogosphere, Steve Bachrach has noted this report in his own blog, where a discussion has opened up on the origins of why carbon can be regarded as abnormal (at […]
A connected world (journals and blogs): The benzene dication. « Henry Rzepa's blog responded on 08 Jul 2015 at 10:44 am #
[…] saw at an ACS conference a year or so earlier. When the article was published, Steve Bachrach blogged about it, noting the claim for pentavalent carbon. The semantics of a valency vs a coordination are subtle, […]