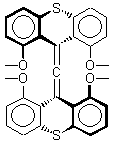
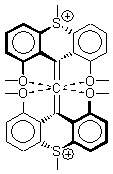
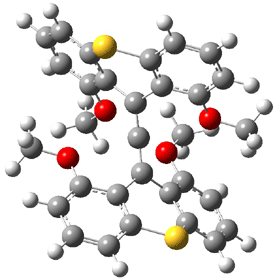
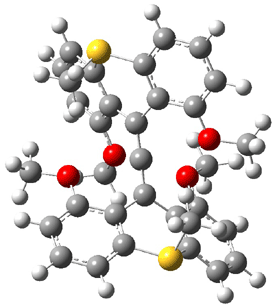
The search for the elusive hypervalent carbon atom took an interesting turn for the positive with the report of the synthesis and characterization of 1 and especially its dication 2.1 The x-ray structure was obtained for both compounds along with computing their B3PW91/6-31G(d) geometries. These computed geometries are shown in Figure 1.
|
|
|
|
1 |
2 |
Figure 1. B3PW91/6-31G(d) optimized geometries of 1 and 2.1
The allene fragment is bent in both structures: 168.5° (169.9° at B3PW91) in 1 and 166.8° (172.7° at B3PW91) in 2. The distances between the central carbon atom of the allene and the four oxygen atoms are 2.66 to 2.82 Å, and computed to be a little longer. Interestingly, these distance contract when the dication 2 is created; ranging from 2.64 to 2.75 Å (again computed to be a little longer). These distances, while significantly longer than normal covalent C-O bonds, are less than the sum of the C and O van der Waals radii. But are they really bonds?
This is not a trivial answer to solve. The authors opt to employ topological electron density analysis (Bader’s atoms-in-molecules approach). Using the electron density from both the high resolution x-ray density map and from the DFT computations, bond paths between the central allene carbon and each oxygen are found, though with, as expected, low values of ρ. The Laplacian of the density at the critical point are positive, indicative of ionic interactions. So according to Bader’s model, the existence of a bond path in a ground state molecule is the necessary and sufficient condition for bonding.
The others conclude by proposing that O…C intermolecular interactions with separations of around 2.6 to 2.8 Å may also suggest hypervalent cases. They note that about 2000 structures in the Cambridge crystallographic database fit this criterion.
References
(1) Yamaguchi, T.; Yamamoto, Y.; Kinoshita, D.; Akiba, K.-y.; Zhang, Y.; Reed, C. A.; Hashizume, D.; Iwasaki, F., "Synthesis and Structure of a Hexacoordinate
Carbon Compound," J. Am. Chem. Soc., 2008, 130, 6894-6895, DOI: 10.1021/ja710423d.
InChIs
1: InChI=1/C31H24O4S2/c1-32-20-9-5-13-24-28(20)18(29-21(33-2)10-6-14-25(29)36-24)17-19-30-22(34-3)11-7-15-26(30)37-27-16-8-12-23(35-4)31(19)27/h5-16H,1-4H3
InChiKey= RJBVLFYVIBCWKW-UHFFFAOYAD
2: InChI=1/C33H30O4S2/c1-34-22-11-7-15-26-30(22)20(31-23(35-2)12-8-16-27(31)38(26)5)19-21-32-24(36-3)13-9-17-28(32)39(6)29-18-10-14-25(37-4)33(21)29/h7-18H,1-6H3/q+2
InChIKey= MVHOSIVPHPDYJJ-UHFFFAOYAM

Egon Willighagen responded on 03 Jun 2008 at 1:35 pm #
Very interesting story! Working on defining a list of atom types, this is really relevant. Now, from a naive organic chemistry perspective, I’d say a chemical bond is something that is stable, that is, that would exist too even it could deform. In the above structures, it seems that the oxygens are forced onto the carbon…
David Bradley responded on 04 Jun 2008 at 2:18 pm #
I’d have to agree, just because you can force two “North” poles of a pair of magnets together, doesn’t mean they’d stick. Nice structural work nevertheless…and maybe one day…
db
sbachrach responded on 04 Jun 2008 at 3:19 pm #
The authors were very careful to call the structure “hexaccordinate” and not “hexavalent”. The later implies chemical bonds, the former some more vague concept of interaction between atom pairs.
Bader argues that the existence of a bond path in a stable structure is the necessary and sufficient conditions for the existence of a bond. I, personally, feel this is an overstatement – or at least requires a redefinition of “bond” to something much weaker than chemists (certainly organic chemists) typically consider. So for example, I published as a post-doc on organolithium complexes and found upwards of 12 bond paths to a single carbon atom! Well, one might consider these to be cation-anion structures and therefore non-directional. A more organic example is the C-N bond in an amide. What is this bond order? The nitrogen is planar and the rotational barrier is larger than about the C-N bond in an alkyl amine? One can talk about cis/trans isomerization here. But it’s not a bond of order 1.5 like in an aromatic compound. Some real organic structures have bond orders that are difficult to fit into simple classification schemes.
I look at the structure of this paper as more than just “atoms jammed together”. The allene is bent to try to move things apart – yet the C…O distance is shorter than van der Waals distances, and there is a bond path in the experimental and theoretical densities. Is this a “bond”? Probably not. Is the carbon “hexavalent”? Probably not. Is is “hexacoordinate”? Probably yes – with some sort of weak stabilizing interaction between the C and O atoms.