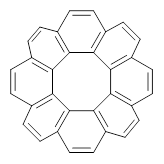
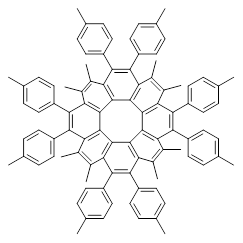
Circulenes are molecules where a central ring is composed of fused benzenoids. Corranulene can also be named [5]circulene and coronene is [6]circulene. In a previous post I discussed the topology of the circulenes. This earlier work suggested that [8]annulene 1 would have a saddle-shape. This hypothesis has now been confirmed with the synthesis of the substituted [8]circulene 2 by Wu and co-workers.1
|
|
|
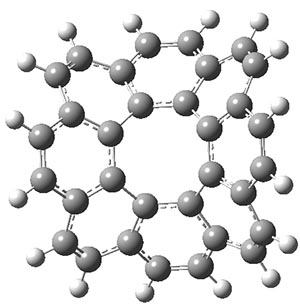
The x-ray structure does show a saddle geometry for 2. The central 8-member ring is tub-shaped, even more puckered that cyclooctatetraene (COT) itself, though the bonds in 2 are nearly of equal length. The bond lengths involving the central carbon atoms appear consistent with an [8]radialene-type structure.
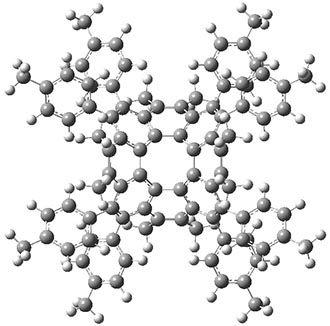
The ωB97X-D/6-31G** optimized geometries of the parent compound 1 and the synthesized compound 2 are shown in Figure 1. These computed structures are very similar to each other, along with being very similar to the x-ray structure of 2.
|
1 |
|
2 |
Figure 1. ωB97X-D/6-31G** optimized geometries of 1 and 2.
(Don’t forget that you can click on these structures – and any other structure on my blog – to interactively manipulate and visualize them, something worth doing here!)
The computed NICS(0) (at HF/6-31+G* – I would really rather have seen these computed with some density functional, preferably at ωB97X-D/6-31G**) values for the six-member rings of both 1 and 2 are negative, ranging from -8.9 ppm to -4.0 ppm, indicating aromatic character. The NICS(0) value at the center of the 8-member ring is +9.8 ppm in 1 and +12.2 ppm in 2. The authors argue that this value cannot discriminate the 8-member ring from that in COT (NICS(0) = 1.98 ppm, the expected value for a non-aromatic ring) and [8]radialene (NICS(0) = -1.2 ppm, also an expected value for a non-aromatic ring). However, they are silent on whether this might actually imply some antiaromatic character to the 8-member ring, which would be consistent with the equivalent bond lengths around the ring.
The authors note that there should be a second isomer of 2 resulting from a flip of the tub. Variable temperature NMR does not show any change in the spectrum, though with a different substituted [8]circulene they do see some coalescence, suggesting a large flipping barrier of at least 20 kcal mol-1. A computational search for this flipping/inversion might be interesting as the transition state is likely to not be planar.
References
(1) Feng, C.-N.; Kuo, M.-Y.; Wu, Y.-T. "Synthesis, Structural Analysis, and Properties of [8]Circulenes," Angew. Chem. Int. Ed. 2013, 52, 7791-7794, DOI: 10.1002/anie.201303875.
InChIs
1: InChI=1S/C32H16/c1-2-18-5-6-20-9-11-22-13-15-24-16-14-23-12-10-21-8-7-19-4-3-17(1)25-26(18)28(20)30(22)32(24)31(23)29(21)27(19)25/h1-16H
InChIKey=BASWMOIVIHXTRC-UHFFFAOYSA-N
2: InChI=1S/C96H80/c1-49-17-33-65(34-18-49)81-73-57(9)58(10)75-83(67-37-21-51(3)22-38-67)85(69-41-25-53(5)26-42-69)77-61(13)62(14)79-87(71-45-29-55(7)30-46-71)88(72-47-31-56(8)32-48-72)80-64(16)63(15)78-86(70-43-27-54(6)28-44-70)84(68-39-23-52(4)24-40-68)76-60(12)59(11)74(82(81)66-35-19-50(2)20-36-66)90-89(73)91(75)93(77)95(79)96(80)94(78)92(76)90/h17-48H,1-16H3
InChIKey=DEKWLSGHBADDAQ-UHFFFAOYSA-N