Sugars comprise a very important class of organic compounds for a variety of reasons, including dietary needs. On the chemical side, their stereochemical variations give rise to interesting
conformational questions. While sugar structures are a well-studied dating back to Fischer, most of these studies are in the solid or solution phase, and these phases can certainly play a role in dictating conformational preferences. This is seen in the differences in conformational distribution with different solvents. Only recently has instrumentation been developed (see these posts for some earlier applications: A, B, C) that can provide structural information of sugars in the gas phase. Cocinero and co-workers describe just such an analysis of fructose.1
Using a combination of laser ablation Fourier transform microwave spectroscopy and quantum chemical computations, they have examined the gas-phase structure of this ketose. There are
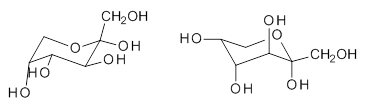
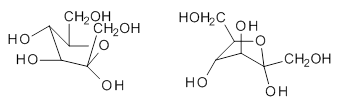
quite a number of important conformational and configurational isomers to consider, as shown in Scheme 1. Fructose can exist in a pyranose form (6-member ring) with the anomeric carbon being α or β. An alternative cyclic form is the 5-member ring furanose form, which again has the two options at the anomeric position. Both the 5- and 6-member rings can ring flip, giving rise to 4 pyranose and 4 furanose forms. Of course there is also the
acyclic form.
Scheme 1. Major Frucotse isomers
|
α-pyranose |
|
|
|
β-pyranose |
|
|
|
α-furanose |
|
|
|
β-furanose |
|
|
|
Open chain |
|
|
The observed microwave spectrum is quite simple, showing evidence of only a single isomer. In comparing the microwave rotational constants and the quartic centrifugal distortion constants with those obtained from MP2 and M06-2x computations, it is clear that the only observed isomer is the β-pyranose isomer in its 2C5 conformation.
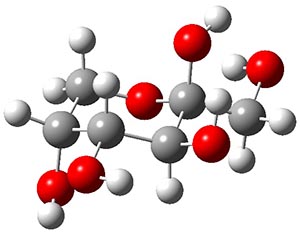
Both MP2 and M06-2x (with a variety of TZ basis sets) predict this isomer to be the lowest energy form by about 2.5 kcal mol-1. This structure is shown in Figure 1. Interestingly, B3LYP predicts the open chain configuration as the most stable isomer, with the β-pyranose isomer about 0.5 kcal mol-1 higher in energy. The authors strongly caution against using B3LYP for any sugars.
Figure 1. MP2/maug-cc-pVTZ optimized structure of β-fructopyranose.
This most stable furanose isomer displays five intramolecular hydrogen bonds that account for its stability over all other possibilities. However, the pyranose form of fructose is very rare in nature, and the Protein Data Bank has only four examples. The furanose form is by far the more commonly found isomer (as in sucrose). Clearly, hydrogen bonding to solvent and other solvent interactions alter the conformational distribution.
References
(1) Cocinero, E. J.; Lesarri, A.; Ecija, P.; Cimas, A.; Davis, B. G.; Basterretxea, F. J.; Fernandez, J. A.; Castano, F. "Free Fructose is Conformationally Locked," J. Am. Chem. Soc. 2013, 135, 2845-2852, DOI: 10.1021/ja312393m.
InChIs:
β-fructopyranose:
InChI=1S/C5H10O6/c6-2-1-11-5(9,10)4(8)3(2)7/h2-4,6-10H,1H2
InChIKey=FFDHYUUPNCCTDA-UHFFFAOYSA-N
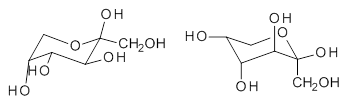
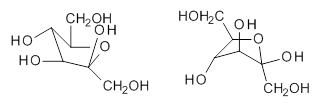

C. Anthony Lewis responded on 20 Mar 2013 at 9:19 am #
Thanks for an interesting post… isn’t there is an -OH missing from the ‘flipped’ pyranose rings in Scheme 1?
Steven Bachrach responded on 21 Mar 2013 at 8:45 am #
Thanks for this catch! – the figure has now been revised.