The Alonso group has once again shown the power of the combination of molecular beam Fourier transform microwave spectroscopy (MB-FTMW) coupled with computations. They examined ephedrine, norephedrine and pseudoephedrine and determined the low energy conformations of each.1 I discuss just the ephedrine case here, but similar results were obtained for the other two compounds.
1
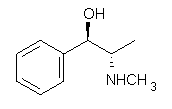
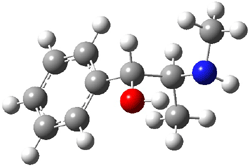
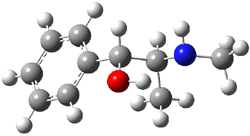
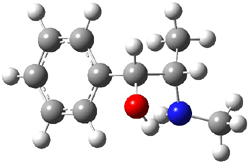
Ephedrine (1) has six potential conformations, differing by the rotation about the C-C bond and the orientation of the methyl group on the nitrogen. They optimized the 6 conformers at MP2/6-311+G(d,p) and corrected the energies for zero-point vibrational energies computed at B3LYP/6-311++G(d,p). The rotational constants and diagonal elements of the 14N quadrupole coupling tensor were computed and obtained by experiment. The comparison of these values (shown in Table 1) made possible the identification of three low energy conformers, labeled as AGa, AGb, and GGa. The structures are shown in Figure 1.
Table 1. Experimental and computeda spectroscopic constants for three conformers of ephedrine.1
|
|
||||||
|
|
AGa |
AGb |
GGa |
|||
|
|
Expt |
Comp |
Expt |
Comp |
Expt |
Comp |
|
A/MHz |
1998.6382 |
2014 |
2115.8768 |
2112 |
1568.2454 |
1566 |
|
B/MHz |
529.5495 |
533 |
503.7943 |
507 |
592.4485 |
597 |
|
C/MHz |
500.1600 |
505 |
475.1734 |
480 |
572.4160 |
579 |
|
χaa/MHz |
2.535 |
2.63 |
2.559 |
2.70 |
2.448 |
2.51 |
|
χbb/MHz |
-2.745 |
-3.26 |
-4.621 |
-4.83 |
-3.205 |
-2.90 |
|
χcc/MHz |
0.210 |
0.63 |
2.062 |
2.14 |
0.7573 |
0.39 |
|
aComputed at MP2/6-311+G(d,p) |
||||||
|
|
||||||
|
AGa |
AGb |
|
GGa |
|
Figure 1. MP2/6-311+G(d,p) computed structures and relative energies (kcal mol-1) of the three conformers of ephedrine.1
The agreement between the experimental and computed spectroscopic values is very good, less than 1.5% for the rotational constants. This excellent agreement makes possible the identification of these three conformers. The experimental population ratio of N(AGa):N(GGa):N(AGb) is 20:4:1, in nice agreement with the computed values. Of structural interest here is the intramolecular O-H…N hydrogen bond in each conformer. The authors also suggest a weak hydrogen bond-like interaction between the N-H and the benzene π-system.
References
(1) Alonso, J. L.; Sanz, M. E.; Lopez, J. C.; Cortijo, V., "Conformational Behavior of Norephedrine, Ephedrine, and Pseudoephedrine," J. Am. Chem. Soc., 2009, 131, 4320-4326, DOI: 10.1021/ja807674q.
InChIs
1: InChI=1/C10H15NO/c1-8(11-2)10(12)9-6-4-3-5-7-9/h3-8,10-12H,1-2H3/t8-,10-/m0/s1
InChIKey=KWGRBVOPPLSCSI-WPRPVWTQBH

Computational Organic Chemistry » Gas—phase structure of fructose responded on 25 Mar 2013 at 7:28 am #
[…] Only recently has instrumentation been developed (see these posts for some earlier applications: A, B, C) that can provide structural information of sugars in the gas phase. Cocinero and co-workers […]