I have written a number of posts discussing long C-C bonds (here and here). What about very long bonds between carbon and a heteroatom? Well, Mascal and co-workers1 have computed the structures of some oxonium cations that express some very long C-O bonds. The champion, computed at MP2/6-31+G**, is the oxatriquinane 1, whose C-O bond is predicted to be 1.602 Å! (It is rather disappointing that the optimized structures are not included in the supporting materials!) The long bond is attributed not to dispersion forces, as in the very long C-C bonds (see the other posts), but rather to σ(C-H) or σ(C-C) donation into the σ*(C-O) orbital.
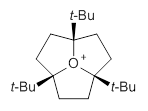
1
Inspired by these computations, they went ahead and synthesized 1 and some related species. They were able to get crystals of 1 as a (CHB11Cl11)– salt. The experimental C-O bond lengths for the x-ray crystal study are 1.591, 1.593, and 1.622 Å, confirming the computational prediction of long C-O bonds.
As an aside, they also noted many examples of very long C-O distances within the Cambridge
Structural database that are erroneous – a cautionary note to anyone making use of this database to identify unusual structures.
References
(1) Gunbas, G.; Hafezi, N.; Sheppard, W. L.; Olmstead, M. M.; Stoyanova, I. V.; Tham, F. S.; Meyer, M. P.; Mascal, M. "Extreme oxatriquinanes and a record C–O bond length," Nat. Chem. 2012, 4, 1018-1023, DOI: 10.1038/nchem.1502
InChIs
1: InChI=1S/C21H39O/c1-16(2,3)19-10-12-20(17(4,5)6)14-15-21(13-11-19,22(19)20)18(7,8)9/h10-15H2,1-9H3/q+1/t19-,20+,21-
InChIKey=VTBHIDVLNISMTR-WKCHPHFGSA-N

Henry Rzepa responded on 08 Jan 2013 at 3:55 pm #
I agree that a blind faith in crystal structures can be dangerous. Ultimately, one has to inspect the (deposited) CIF file (using a text editor) for each structure to be sure, and you probably have to be a crystallographer to know what to look for.
I use the following simple rules when searching Cambridge for specific geometric ranges.
1. R factor less than 0.05
2. Not disordered
3. No errors
4. Measurement less than 150K.
When this is done for C-O bonds for O with three other bonded atoms, over the distance range 1.58 to 1.7 A, only six hits come up in this range. All six have one metal bonded to O, and none is an oxonium cation. So Mascal and co do seem to have found something quite unique.