Compounds with long C-C bonds have typically been designed by placing large, sterically bulky groups attached to the two carbons. Not only does this lead to a longer bond (like the 1.67 Å C-C bond in 1) but these bulky groups also weaken the bond. This leads to molecules that tend to be difficult to isolate.
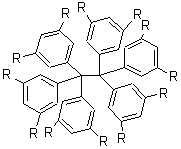
1 R = t-But
Schreiner has taken an alternative approach: design a sterically crowded molecules that is stabilized by dispersive forces between the large groups!1 The dimer formed from diamantane 2 was prepared and isolated. The C-C distance is quite long: 1.647 Å. The compound is stable up to at least 300 ° C.
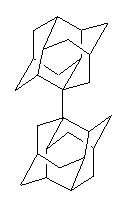
2
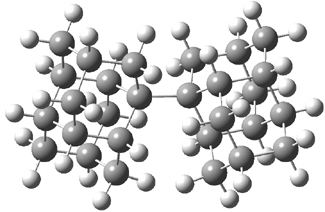
Computations of 2 were performed with a variety of density functional, all of which predict a long C-C bond. The bond dissociation energy of 2 is predicted to be 43.9 kcal mol-1 at B3LYP/6-31G(d,p), a value consistent with the long CC bond. However, B3LYP does not account for dispersion. The H…H distances between the two diamantyl groups range from 1.94 – 2.28 Å, suggesting that there could be appreciable dispersion stabilization. In fact, computing the BDE with B3LYP+D (Grimme’s dispersion correction) or B97D or M06-2x (all of which account for dispersion to some extent), predicts a much stronger bond, with the BDE ranging from 65-71 kcal mol-1! Here is a stable molecule with a stroing, yet long C-C bond – where a good deal of the strength results not form the interaction between the two atoms of the formal bond, but rather from the energy associated form interactions across the entire molecule. This is a true delocalization effect!
Figure 1. B3LYP-D/6-31G(d,p) optimized structure of 2.
References
(1) Schreiner, P. R.; Chernish, L. V.; Gunchenko, P. A.; Tikhonchuk, E. Y.; Hausmann, H.; Serafin, M.; Schlecht, S.; Dahl, J. E. P.; Carlson, R. M. K.; Fokin, A. A., "Overcoming lability of extremely long alkane carbon-carbon bonds through dispersion forces," Nature, 2011, 477, 308-311, DOI: 10.1038/nature10367.
InChIs
1: InChI=1/C86H126/c1-73(2,3)55-37-56(74(4,5)6)44-67(43-55)85(68-45-57(75(7,8)9)38-58(46-68)76(10,11)12
,69-47-59(77(13,14)15)39-60(48-69)78(16,17)18)86(70-49-61(79(19,20)21)40-62(50-70)80(22,23)24,71-51
-63(81(25,26)27)41-64(52-71)82(28,29)30)72-53-65(83(31,32)33)42-66(54-72)84(34,35)36/h37-54H,1-36H3
InChIKey=SFCRGHMFOGXDMZ-UHFFFAOYAS
2: InChI=1/C28H38/c1-13-7-23-19-3-15-4-20(17(1)19)24(8-13)27(23,11-15)28-12-16-5-21-18-2-14(9-25(21)28)
10-26(28)22(18)6-16/h13-26H,1-12H2
InChIKey=MMYAZLNWLGPULP-UHFFFAOYAU

Henry Rzepa responded on 02 Nov 2011 at 1:09 am #
I think by far the most interesting contribution comes from the 12 H…H interactions between the two adamantyl groups. These measure out at around 2.1-2.2Å. The sum of the vdW radii between two Hs is 2.4Å (which I have always felt is rather too long). But, at ~ 0.2-0.3 kcal each for such an interaction, the effect still seems too small to account for a 20 kcal/mol strengthening for the molecule as a whole?
Steven Bachrach responded on 02 Nov 2011 at 7:13 am #
I have always thought about dispersion as being related to the surface area in contact between the two species. That surface area might not be linearly related tot he number of H…H interactions.
Henry Rzepa responded on 02 Nov 2011 at 8:35 am #
Well, its a 1/r^^6 distance dependence, and that dies off pretty quickly, does it not? I would imagine that only H…H contacts less than 2.4Å would contribute significantly to the overall stability? Now if it was electrostatic, then yes that dies off very slowly. But this is dispersion we are talking about, and for this (relatively small) molecule, the effect seems to be 20+ kcal. OK, for enzymes, with 1000s of atoms, but surely not for something this small.
That said, I have to say we have not done a B3LYP study for a little while now, preferring the dispersion (and solvation) corrected functionals for pretty much everything.
Lukas Sobczak responded on 25 Nov 2011 at 7:52 am #
Compound 1 is a bad example, because it exist. If you remove tBu (R=H), such molecule doesn’t exist, dimer had different structure (imagine two Ph3C radicals, central C bonds Phenyl (Para position) of second molecule, (Ph3C-C6H5=CPh2).) And those radicals exist in RT in solution in quite high concentration. It was suggested that 1 is stbilized by dispersive forces too.
By the way, Is it possible to calculate conformations of organic molecules under high pressure, preferably in solution? I have Diels–Alder products in mind. Qualitative results are ok.
Which potentials should I use to predict energy of ion-neutral molecules associoates in solution (chloride or acetate anion with amides and/or ureas)?
Thanks for answers 🙂
Michael Mauksch responded on 07 Dec 2011 at 10:06 am #
I’d like to point out that “being stable” is a relative, rather than absolute property of a molecule: a molecule might be very energy rich, but unless a facile escape route towards a lower energy form opens up, it could still exist.
Gomberg’s triphenylmethyl radical is stabilized because the radical electron is delocalized into the nearly co-planar phenyl rings, preventing dimerization to the hypothetical hexaphenylethane.
Such an easy escape route is not available for compound 1, though, because the tert-butyl groups hinder a co-planar arrangement of the phenyl rings.
ITOH Shuhei responded on 20 Dec 2011 at 6:53 am #
The BDE of ethane is 72.7, 77.2 kcal/mol at b3lyp/6-31+G(d,p), M06-2X/6-31+G(d,p).
The difference of BDE indicates difference of radical stability for computational levels.
I think that quantitatively comparing is no meaning.
Computational Organic Chemistry » The longest straight chain alkane responded on 10 Sep 2012 at 8:13 am #
[…] chemistry, both structure and reactivity, is truly coming into prominence (see for example this blog post for a compound whose stability is the result of dispersion). This has been driven in part by new computational techniques to properly account for dispersion. […]
What is the range of values for a (sp3)C-C(sp3) carbon single bond? « Henry Rzepa responded on 12 Sep 2012 at 9:11 am #
[…] (in which both carbon termini are sp3 hybridised)? I pose this question since Steve Bachrach has posted on how to stabilize long bonds by attractive dispersive interactions, and more recently commenting […]
Computational Organic Chemistry » Dispersion leads to long C-C bonds responded on 25 Sep 2012 at 7:28 am #
[…] on his previous paper1 regarding alkanes with very long C-C bonds, which I commented upon in this post. He and his colleagues report2 now a series of additional diamond-like and adamantane-like […]
Antwan responded on 01 Nov 2018 at 8:23 am #
I do not know whether it’s just me or iff perhaps everyone else encountering issues
with your site. It looks like some of the written trxt on your
content are running offf the screen. Can somebody else please provide feedback and let me
know iff this is happening to them as well? This could be a
problem with my browser because I’ve had this happen before.
Cheers