Can a hydrogen bonding network affect acidity? Kass has examined the polyol 1 whose conjugate base 1cb can potentially be stabilized by a large hydrogen bonding network.1 Kass had previously found a significant acidy enhancement in comparing t-butanol (ΔG(deprotonation) = 369.2 kcal mol-1) with that of 2 (ΔG(deprotonation) = 334.4 kcal mol-1).2
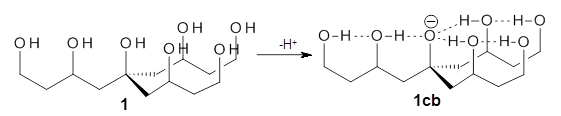
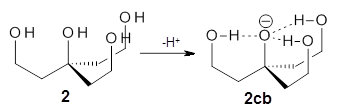
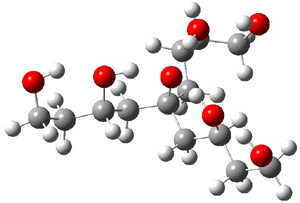
Table 1 lists the computed and experimental free energies of deprotonation of 1. The experimental values are computed at M06-2x/maug-cc-pVT(+d)Z. The structure of 1cb is drawn in Figure 1.
Table 1. Computed and experimental free energies (kcal mol-1 of deprotonation of some alcohols.
|
|
MO6-2x |
Expt |
|
t-butanol |
368.6 |
369.3 |
|
2 |
335.0 |
334.4 |
|
1 |
320.2 |
313.5 |
The difference in the acidity of t-butanol and 2, some 30 kcal mol-1, reflects the stability afforded by three intramolecular hydrogen bonds to the oxyanion. In going from 2cb to 1cb, each of the hydroxyl groups that donate to the oxyanion act as the acceptor of a hydrogen bond from the more removed hydroxyl groups. There is in effect a first and second layer of hydrogen bond network in 1cb. These secondary hydrogen bonds lead to further stabilization of the anion, as reflected in the diminished DPE of 1 over 2: 320.2 vs. 335.0 kcal mol-1. Note that this secondary layer does not stabilize the anion to the same degree as the primary layer, but nonetheless its effect is large and quite striking.
|
1cb |
Figure 1. M06-2x/maug-cc-pVT(+d)Z optimized structure of 1cb.
Even in solution these more remote hydrogen bonds can stabilize the anion. So, using the CPCM approach and modeling DMSO, 2 is predicted have a pKa that is 15 units below that of t-butanol, and 1 is predicted to be 3 pKa units more acidic than 2. Experiments verify this prediction with the pKas of 16.1 for 2 and 11.4 for 1.
References
(1) Shokri, A.; Abedin, A.; Fattahi, A.; Kass, S. R. "Effect of Hydrogen Bonds on pKa Values: Importance of Networking," J. Am. Chem. Soc. 2012, 134, 10646-10650, DOI: 10.1021/ja3037349
(2) Tian, Z.; Fattahi, A.; Lis, L.; Kass, S. R. "Single-Centered Hydrogen-Bonded Enhanced Acidity (SHEA) Acids: A New Class of Brønsted Acids," J. Am. Chem. Soc. 2009, 131, 16984-16988, DOI: 10.1021/ja9075106
InChIs
1: InChI=1S/C13H28O7/c14-4-1-10(17)7-13(20,8-11(18)2-5-15)9-12(19)3-6-16/h10-12,14-20H,1-9H2
InChIKey=HGTVPOTWAYDRSM-UHFFFAOYSA-N
1cb: InChI=1S/C13H27O7/c14-4-1-10(17)7-13(20,8-11(18)2-5-15)9-12(19)3-6-16/h10-12,14-19H,1-9H2/q-1
InChIKey=UQPPTNIHRICITD-UHFFFAOYSA-N
2: InChI=1S/C7H16O4/c8-4-1-7(11,2-5-9)3-6-10/h8-11H,1-6H2
InChIKey=FAQWYKIIWYVDPQ-UHFFFAOYSA-N
2cb: InChI=1S/C7H15O4/c8-4-1-7(11,2-5-9)3-6-10/h8-10H,1-6H2/q-1
InChIKey=WSCPTRIAWKZJFZ-UHFFFAOYSA-N

Henry Rzepa responded on 04 Sep 2012 at 7:24 am #
This links in nicely to my previous comment here. I was arguing that the presence of additional components (for example explicit hydrogen bonds, and counter-ions) might play a role in controlling the outcome of thiolate attack on a dichlorobutenone, as reported by Dan Singleton and blogged by Steve here. If such species can influence a pKa, why not a reaction outcome?
Dan actually replied to this, disagree(ing) with (my) blog posts on multiple levels, arguing in turn that the presence of (sodium or other) counter-ions makes no difference and that their inclusion could be regarded as “nonphysical”. You might be interested that my replies to Dan’s comments (here) evaluated the reaction which Dan had subjected to direct reaction dynamics by including both explicit hydrogen bonding and the counter-ion. Remarkably, the reaction transition state is stable to the presence of these extra components however they are arranged geometrically (I evaluated four different geometric models), and is instead robustly controlled by intrinsic stereoelectronic affects, which appear to be un-influenced by the presence of these additional components. That does appear to be a physical (and not an unphysical) conclusion.
I have nevertheless challenged Dan to show that the same is true of the computed reaction dynamics (i.e. that they too are not changed by the presence of hydrogen bonds or counter-ion, or indeed more technical issues such as choice of density functional or basis set). If this too is shown then I think we would have a nicely robust instance of reaction control via dynamics rather than transition state alone. But of course it could not be used to generalize this statement, i.e. that the presence of hydrogen bonds and counter-ions does not influence reaction dynamics (as it appears to do with pKa, as Steve has discussed above).
Finally, the species 1cb and 2cb above are both computed for equation (1) in the article itself as anions. That is to say, from what I can tell, the calculations have been carried out for a system with overall charge of -1. There is no counter-ion present to make the overall system carry a zero charge. The results, as Steve indicates above, are impressive, but not perfect. Thus for 1, there is a discrepancy of about 7 kcal/mol. Again, one wonders what result the inclusion of a counter-ion might give. One will not know if it improves matters or not unless one investigates!