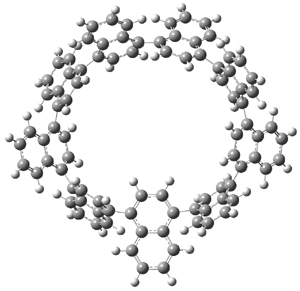
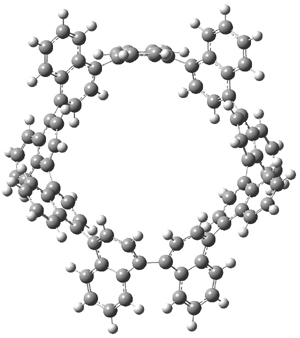
Itami continues to design novel macrocycles containing aromatic rings (see this post). This latest paper reports the synthesis of the first nanohoops containing naphthylenes, namely [9]cyclo-1,4-naphthylene 1.1 Since the macrocycle contains an odd number of naphthylene units, the lowest energy conformation is of C2 symmetry with one of the naphthylene rings in the plane of the macrocycle. (See Figure 1 for the B3LYP/6-31G(d) optimized structure). This conformation gives rise to 27 peaks in the proton NMR, and while the value of the computed chemical shifts differ from the experimental ones by about 0.5 to 1 ppm, their relative ordering is in very nice agreement.
|
1 |
2 |
Figure 1. B3LYP/6-31G(d) optimized geometries of 1 and the racemization transition state 2.
Itami also notes that 1 is chiral and computed the barrier for racemization of 19.9 kcal mol-1¸ through the transition state 2, also shown in Figure 1. This racemization process is compared with the racemization of 1,1’-binaphthyl.
References
(1) Yagi, A.; Segawa, Y.; Itami, K., "Synthesis and Properties of [9]Cyclo-1,4-naphthylene: A π-Extended Carbon Nanoring," J. Am. Chem. Soc. 2012, 134, 2962-2965, DOI: 10.1021/ja300001g
InChIs
1: InChI=1/C90H54/c1-2-20-56-55(19-1)73-37-38-74(56)76-41-42-78(60-24-6-5-23-59(60)76)80-45-46-82(64-28-10-9-27-63(64)80)84-49-50-86(68-32-14-13-31-67(68)84)88-53-54-90(72-36-18-17-35-71(72)88)89-52-51-87(69-33-15-16-34-70(69)89)85-48-47-83(65-29-11-12-30-66(65)85)81-44-43-79(61-25-7-8-26-2(61)81)77-40-39-75(73)57-21-3-4-22-58(57)77/h1-54H/b75-73-,76-74-,79-77-,80-78-,83-81-,84-82-,87-85-,88-86-,90-89-
InChIKey=WUFIQFYLEOBLMY-YNQZQJAJBX