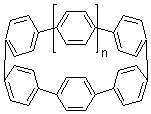
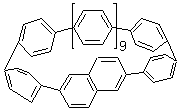
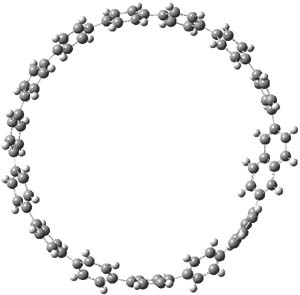
Single-walled carbon nanotubes (SWNT) can be thought of as built from component macrocycles, often called nanohoops. So, for example, cycloparaphenylenes like 1 can be the thought of as the precursor (at least in principle) of armchair SWNTs. To create chiral SWNTs, Itami1 has suggested that cycloparaphenylene-naphthalene (2) and other acene substituted macrocycles would serve as appropriate precursors.
|
|
|
Itami has synthesized 2 (having 13 phenyl groups and one naphthyl group) and also examined the ring strain energy and racemization energy of a series of these types of compounds at B3LYP/6-31G(d). As might be expected, based on studies of the cycloparaphenylenes themselves,2,3 ring strain energy decreases with increasing size of the macrocycle. So, for example, the macrocycle with one naphthyl group and 5 phenyl rings has a strain energy of 90 kcal mol-1, but the strain is reduced to 40 kcal mol-1 with 13 phenyl rings.
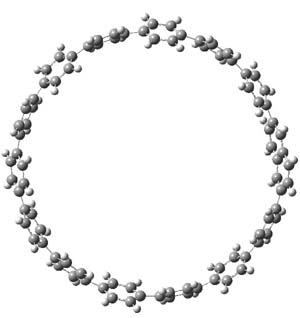
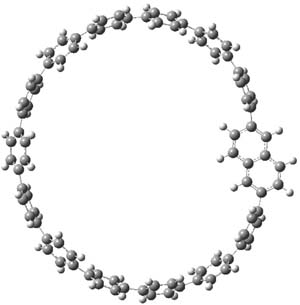
The macrocycle 2 and related structures are chiral, existing in P and M forms. The racemization involves first rotation of the naphthyl group, as shown in Figure 1, with a barrier of about 8 kcal mol-1. The direct product has the opposite stereochemistry but is not in the lowest energy conformation. Rotations of some phenyl groups remains to occur, but these rotations are expected to have a barrier less than that for the rotation of the naphthyl group, based on the previous study of cycloparaphenylenes. Again, the racemization barrier decreases with the size of the macrocycle.
|
(P)-2 |
2-TS |
|
(M)-2’ |
|
Figure 1. B3LYP/6-31G(d) optimized structures along the racemization pathway of 2.
References
(1) Omachi, H.; Segawa, Y.; Itami, K., "Synthesis and Racemization Process of Chiral Carbon Nanorings: A Step toward the Chemical Synthesis of Chiral Carbon Nanotubes," Org. Lett., 2011, 13, 2480-2483, DOI: 10.1021/ol200730m
(2) Segawa, Y.; Omachi, H.; Itami, K., "Theoretical Studies on the Structures and Strain Energies of Cycloparaphenylenes," Org. Lett., 2010, 12, 2262-2265, DOI: 10.1021/ol1006168
(3) Bachrach, S. M.; Stuck, D., "DFT Study of Cycloparaphenylenes and Heteroatom-Substituted Nanohoops," J. Org. Chem., 2010, 75, 6595-6604, DOI: 10.1021/jo101371m
InChIs
2: InChI=1/C88H58/c1-2-60-4-3-59(1)61-5-9-63(10-6-61)65-13-17-67(18-14-65)69-21-25-71(26-22-69)73-29-33-75(34-30-73)77-37-41-79(42-38-77)81-45-49-83(50-46-81)85-53-55-88-58-86(54-56-87(88)57-85)84-51-47-82(48-52-84)80-43-39-78(40-44-80)76-35-31-74(32-36-76)72-27-23-70(24-28-72)68-19-15-66(16-20-68)64-11-7-62(60)8-12-64/h1-58H/b61-59-,62-60-,65-63-,66-64-,69-67-,70-68-,73-71-,74-72-,77-75-,78-76-,81-79-,82-80-,85-83-,86-84+
InChIKey=VXOGKWSXPGSUSO-ZMOMEJFTBU

Henry Rzepa responded on 02 Jun 2011 at 3:52 am #
I speculate on what the chiroptical properties of such systems might be (if they were stable to racemisation). Also of interest is all-carbon versions, which might look like this. Thee are described as having colossal paramagnetic moments, and no doubt equally interesting chiroptical properties.
Metallic carbon nanotori « Henry Rzepa responded on 02 Jun 2011 at 4:19 am #
[…] e.g. its optical rotation would also be colossal! This post, by the way, was induced by seeing Steve Bachrach’s fascinating exploration of chiral […]
Computational Organic Chemistry » Fantastic host-guest complex responded on 13 Sep 2011 at 8:40 am #
[…] (Check out this post for other interesting nanohoops.) […]
Bryan M. Wong responded on 12 Oct 2011 at 2:47 pm #
The excited-state properties of these unique chiral nanorings exhibit very interesting optoelectronic properties…especially one ring, which shows very large photoinduced transitions:
Wong, B. M.; Lee, J. W., “Anomalous Optoelectronic Properties of Chiral Carbon Nanorings…and One Ring to Rule Them All,” J. Phys. Chem. Lett., 2011, 2, 2702-2706, DOI: 10.1021/jz2012534
http://pubs.acs.org/doi/full/10.1021/jz2012534
Dennis Bayne responded on 22 Jan 2012 at 8:12 pm #
1) How would one go about modelling a nanotorus with over 250K atoms?
2) How would one go about assembling such a device for nanopennies-a-piece?
Steven Bachrach responded on 23 Jan 2012 at 8:36 am #
Check out my paper, where we computed the structures of nanohoops having up to 24 phenyl rings (194 atoms)
DFT Study of Cycloparaphenylenes and Heteroatom-Substituted Nanohoops
Steven M. Bachrach and David Stück
J. Org. Chem., 2010, 75 (19), pp 6595–6604
DOI: 10.1021/jo101371m
Dennis Bayne responded on 14 Feb 2012 at 12:58 pm #
Steve, thats a little over 4.5 thousand atoms. I’m talking about 0.25 million atoms.
How would you go about modelling such a monstrocity, let alone self-assemble it?
Computational Organic Chemistry » Nanohoop of linked napthlylene groups responded on 27 Mar 2012 at 2:30 pm #
[…] continues to design novel macrocycles containing aromatic rings (see this post). This latest paper reports the synthesis of the first nanohoops containing naphthylenes, namely […]
Robert Gotwals responded on 01 Jul 2012 at 4:31 am #
Is there a downloadable structure file for the 9-membered benzene nanohoop AND/OR the 9-membered naphthalene nanohoop? Optimized, preferably, and MOL or PDB format.