In a follow-up to their experimental study that found that cyclobutenone is an excellent dienophile (and which I blogged about here), Danishefsky teams up with Houk and provides an insight into the reactivity.1 In the Diels-Alder reaction of cyclopentadiene with 2-cyclohexenone, 2-cyclopentenone and cyclobutenone, the product yield increases in the order 36%, 50% and 77%. M06-2x activation enthalpies decrease in this series 15.0, 13.3 and 10.5 kcal mol-1.
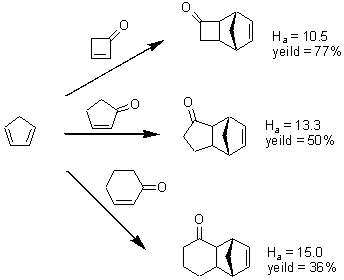
While these activation energies do not correlate with reaction energies, the activation energies do correlate nicely with the distortion energy. (Distortion energy is the energy required to distort reactants to their geometries in the transition state, but without their interaction.) Houk and Danishefsky argue that it is much easier to distort cyclobutenone to its geometry in the TS (and this distortion is primarily moving the alkenyl hydrogens out of plane, away from the incoming diene) than for the larger rings. This is due to (a) the larger s-character of the C-H bond in the smaller ring and (b) the C-C-C angle in the smaller ring is closer to the angle in the pyramidalized TS structure.
References
(1) Paton, R. S.; Kim, S.; Ross, A. G.; Danishefsky, S. J.; Houk, K. N., "Experimental Diels–Alder Reactivities of Cycloalkenones and Cyclic Dienes Explained through Transition-State Distortion Energies," Angew. Chem. Int. Ed., 2011, 44, 10366–10368, DOI: 10.1002/anie.201103998

luysii responded on 08 Nov 2011 at 10:10 pm #
Looks like cyclobutENone rather than cyclobutanone. Am I missing something?
Steven Bachrach responded on 09 Nov 2011 at 5:09 pm #
Thanks for the catch – I have corrected this in the post
Computational Organic Chemistry » Review of the Activation Strain/Distortion-Interaction Model responded on 16 Oct 2017 at 9:54 am #
[…] a broad range of applications, including SN2 and E2 reactions, pericyclic reactions (including Diels-Alder reactions of enones and the dehdydro Diels-Alder reaction that I have discussed in this blog), a click reaction, a few […]