Li and Danishefsky report a study of the Diels-Alder reaction involving cyclobutenone 1 as the dienophile.1 They claim that “perhaps the ring strain of 1 might well serve to enhance its dienophilicity relative to corresponding cyclopentenones or cyclohexenones.” In fact, 1 is an excellent dienophile, with reactions at or below 0° being accomplished in less than half a day with yields upwards of 90%. The reaction goes with endo selectivity.
What is surprising to me is the statement in the article:
While the magnitude of the effect could not have been predicted in advance, the rate enhancement with 1 must reflect the favorable effects of rehybridization of two particularly strained sp2 carbons in the cycloaddition transition state.
Now, Danishefsky alludes to upcoming computations results in a future paper, but I don’t see why the rate enhancement could not have been “predicted in advance”. So, I have optimized the structures of reactants, endo and exo transition states, and products of the reaction of 1,3-butadiene with 1, cyclopentenone 2 and cyclohexenone 3 at B3LYP/6-311G(d) – Reactions 1-3.
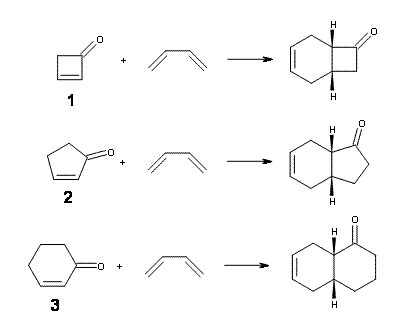
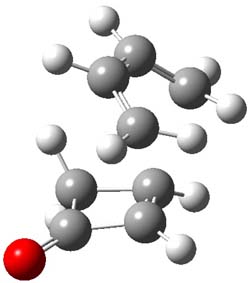
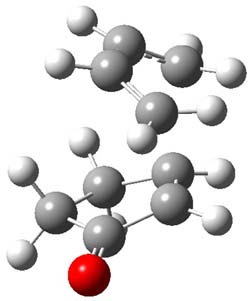
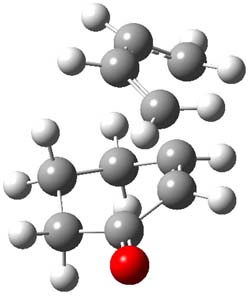
The endo TS is preferred for the reaction of 1 and 2, while the endo and exo TSs for 3 are essentially isoenergetic. The optimized geometries are shown in Figure 1.
|
1TSendo |
2TSendo |
3TSendo |
Figure 1. B3LYP/6-311G(d) optimized geometries of the endo TSs of Reactions 1-3.
The computed activation barriers and overall reaction energies are listed in Table 1. Clearly, the cycloaddition of 1 is favored both in terms of kinetics (having the lowest barrier) and thermodynamically (having the most exothermic reaction energy). In fact, the reaction barriers increases in going from 1 to 2 to 3 and the exothermicity decreases in that same order. This nicely dovetails with the strain energies of the dienophiles and the fact that cyclopententones and cyclohexenones are generally poor dienophiles. Thus, one clearly could have predicted these results in advance!
Table 1. Activation and Reaction Energy (kcal mol-1) for Reactions 1-3.
|
Reaction |
Ea |
ΔE |
|
1 |
18.8 |
-35.2 |
|
2 |
24.1 |
-27.1 |
|
3 |
25.7 |
-27.1 |
Nonetheless, the experimental work is extremely nice and this work offers a new avenue into some interesting bicyclic structures.
Note: This post has been modified to correct the errors in the product structures and their associated InChIs and InChIKeys.
References
(1) Li, X.; Danishefsky, S. J., "Cyclobutenone as a Highly Reactive Dienophile: Expanding Upon Diels-Alder Paradigms," J. Am. Chem. Soc., 2010, 132, 11004-11005, DOI: 10.1021/ja1056888
InChIs
1: InChI=1/C4H4O/c5-4-2-1-3-4/h1-2H,3H2
InChIKey=DFLRGCFWSRELEL-UHFFFAOYAP
1prod: InChI=1/C8H10O/c9-8-5-6-3-1-2-4-7(6)8/h1-2,6-7H,3-5H2/t6-,7-/m0/s1
InChIKey=AYXQRXAAJYZWJJ-BQBZGAKWBC
2: InChI=1/C5H6O/c6-5-3-1-2-4-5/h1,3H,2,4H2
InChIKey=BZKFMUIJRXWWQK-UHFFFAOYAH
2prod: InChI=1/C9H12O/c10-9-6-5-7-3-1-2-4-8(7)9/h1-2,7-8H,3-6H2/t7-,8-/m0/s1
InChIKey=LOJATDUUSCWAOA-YUMQZZPRBU
3: InChI=1/C6H8O/c7-6-4-2-1-3-5-6/h2,4H,1,3,5H2
InChIKey=FWFSEYBSWVRWGL-UHFFFAOYAT
3prod: InChI=1/C10H14O/c11-10-7-3-5-8-4-1-2-6-9(8)10/h1-2,8-9H,3-7H2/t8-,9-/m0/s1
InChIKey=LFDGSLNQYSSFGI-IUCAKERBBQ

Mrinmoy Nag responded on 26 Aug 2010 at 11:35 pm #
Dr. Bachrach,
Double bonds in the product structures are missing. I love your blog and have started reading your wonderful book.
Thanks,
Mrinmoy
Steven Bachrach responded on 27 Aug 2010 at 8:11 am #
Mrinmoy,
Thanks for catching the errors in the product structures. I have updated the post to reflect the changes necessary.
And thanks for the nice comments about the blog and Book!
Steven
Henry Rzepa responded on 30 Aug 2010 at 1:19 am #
Very nice result Steve; it exemplifies I think how e.g. blogs can help enhance or clarify experimental results, on a timescale quite different from traditional publication methods. Blogs already have a reputation for rapidly picking up/exposing errors, but I would like to argue that they have other functions to perform, and this post by Steve illustrates this nicely.
I have one question and one observation.
1. If you compute the entropy, you can then use e.g. Ln (k/T) = 23.76 – ΔG/RT to compute an approximate rate for the reaction, and hence compare the acceleration with ring size. In this regard, ΔH, ΔS and ΔG seem more useful than Ea (?). Who knows, there might be some surprises with the entropies for this series, since a lot of vibrations (which contribute to the partition functions) change as the rings get less strained.
2. I did something related on a recent blog in computing an approximate lifetime for 1,3-cyclobutadiene and carbon dioxide under various conditions (including proximity to an acid catalyst inside a calixarene host), in the light of the recent appearance of an article on the topic in Science. In this case, I think again, it could have been predicted what the lifetime of that pair might be (at least approximately, within the constraints of Eyring transition state theory). There issue there was to try to predict if these two small molecules could co-exist for long enough to have their X-ray structure determined as separate species (i.e. asking the question: were they unreactive enough in a Diels-Alder reaction to be so studied).
Computational Organic Chemistry » Distortion energy and the Diels-alder reaction responded on 08 Nov 2011 at 1:49 pm #
[…] study that found that cyclobutanone is an excellent dienophile (and which I blogged about here), Danishefsky teams up with Houk and provides an insight into the reactivity.1 In the Diels-Alder […]