There are three generic conformations of α-amino acids in the gas phase: A-C. These are stabilized by intramolecular hydrogen bonds. While computations suggest that all three are close in energy, the very detailed laser ablation- molecular beam-Fourier transform microwave (LA-MB-FTMW) experiments of the Alonso group (mentioned in these previous posts: guanine, cysteine, ephedrine) have identified only the first two conformations. Cooling of the structures in the jet expansion appears to be the reason for the loss of the (slightly) higher energy conformer C.

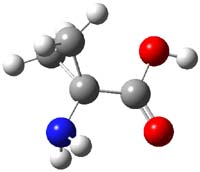
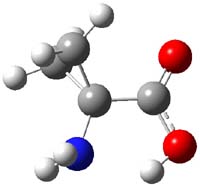
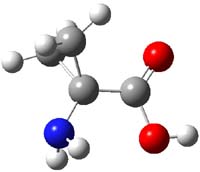
Alonso now reports on the structure of 1-aminocyclopropanecarboxylic acid 1.1 The three MP2/6-311+G(d,p) optimized conformation are shown in Figure 1. The interaction between the cyclopropyl orbitals and the carbonyl π-bond suggests that only two structures (where the carbonyl bisects thecyclopropyl plane) will exist and that rotation between them may require passage through a prohibitively high barrier. In fact, computations suggest a barrier of 2000 cm-1 (5.7 kcal mol-1). This is much larger than the typical rotation barrier of the amino acids that interconvert A with C, which are about 400 cm-1 (1 kcal mol-1).
|
1A |
1B |
1C |
After careful examination of the microwave spectrum, all three conformations 1A-C were identified by comparing the experimental value of the rotational constants, and 14N nuclearquadrupole coupling constants with the computed values. Really excellent agreement is found, including in the ratio of the relative amounts of the three isomers. Once again, we have an exquisite example of the importance of computations and experiments being used in conjunction to solve interesting chemical problems.
References
(1) Jimenez, A. I.; Vaquero, V.; Cabezas, C.; Lopez, J. C.; Cativiela, C.; Alonso, J. L., "The Singular Gas-Phase Structure of 1-Aminocyclopropanecarboxylic Acid (Ac3c)," J. Am. Chem. Soc., 2011, 133, 10621-10628, DOI: 10.1021/ja2033603
InChIs
1: InChI=1/C4H7NO2/c5-4(1-2-4)3(6)7/h1-2,5H2,(H,6,7)/f/h6H
InChIKey=PAJPWUMXBYXFCZ-BRMMOCHJCR