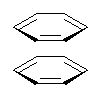
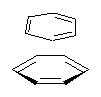
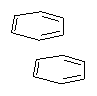
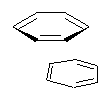
Yet more on the benzene dimer. Lesczynski has optimized 9 different benzene dimer configurations, shown in Scheme 1.1 There are two T-shaped isomers, where a hydrogen from one benzene interacts with the center of the π-cloud of the second. There are two bent versions of the T-shape, called Bent-T-shape. There are two sandwich configurations and two variants where the benzenes are parallel but displaced. Lastly, they report on a new variant, the V-shape configuration. (Once again, the author has not deposited the structures and so I can’t produce interactive figures!)
Scheme 1
|
|
|
|
|
|
|
|
|
|
|
|
The structures were optimized at MP2/aug-cc-pVDZ and then single point energies computed at MP4(SDTQ)/aug-cc-pVDZ and corrected for basis set superposition error. I list these energies in Table 1. They authors note that in comparison with CCSD(T) computations one has to adjust the amount of BSSE correction – which just supports my long-held contention that the standard counterpoise correction overcompensates and that we really have no reliable way of correcting for BSSE.
Table 1. Dimerization energies (kcal mol-1) at MP4(SDTQ)/aug-cc-pVDZ.1
|
T-1 |
T-2 |
BT-1 |
BT-2 |
SW-1 |
SW-2 |
PD-1 |
PD-2 |
V |
The relative energies of the 9 configurations are similar, indicating a very flat potential energy surface. The lowest energy structure is BT-2, and the V-shape configuration is the least favorable of the nine geometries examined.
References
(1) Dinadayalane, T. C.; Leszczynski, J., "Geometries and stabilities of various configurations of benzene dimer: details of novel V-shaped structure revealed " Struct. Chem. 2009, 20, 11-20, DOI: 10.1007/s11224-009-9411-6.
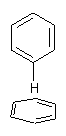
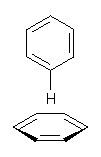
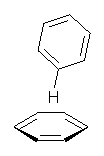
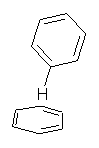

Henry Rzepa responded on 02 Jun 2009 at 8:31 am #
The packing of benzene seems a problem of perpetual interest to some. But I would like to pose some questions
1. Why is the melting point of benzene about 110C higher than cyclohexadiene?
2. Why is it almost the same as that of cyclohexane itself?
Do these detailed interaction studies of benzene with itself cast light on the above properties?
Rob Paton responded on 03 Jun 2009 at 3:34 am #
The second question posed above has been also been asked by Stefan Grimme (Angew. Chem. Int. Ed. 2008, 47, 3430).
In that article, he notes that whilst benzene and cyclohexane share similar melting points, the heat of vaporization is larger for napthalene than decalin by 5 kcal/mol – it is only for aromatics with more than ten carbon atoms that binding is stronger than a similarly-sized unsaturated molecule, as the (favourable) dispersion interaction is greater and the unfavourable Pauli exhange repulsion is smaller.
Thanks for all the work that goes in to this blog!
Steven Bachrach responded on 03 Jun 2009 at 4:30 am #
@Rob – I have written about the Grimme paper you mention at this post.
Thanks for the kind words as well.
Computational Organic Chemistry » Benzene dimer once again responded on 12 Oct 2009 at 7:40 am #
[…] more into the benzene dimer (see these previous posts: “Benzene dimer again“, “Benzene dimer“, “π-π stacking (part 2)“, “π-π […]
Quora responded on 22 Oct 2011 at 11:02 am #
What are some concrete examples where the addition of an extra coordinate/variable helped break a degeneracy?…
This happens all the time in chemical reactions. The classic example is benzene dimer, which is a stable molecule weakly bound by van der Waals dispersion. If you take two benzenes and align their rings in exactly the same orientation (what in chemistr…