Macrocycles composed of aromatic subunits, like polycycloparaphenylenes, are of interest as components of nanotubes and for possible interesting optical properties. Tremendous advances have occurred over the past decade in preparing these rings ; see for examples these posts. Yamago now reports on the synthesis, optical properties and structure of [4]cyclo-2,7-pyrenylene 1, made by joining four pyrene units together.1
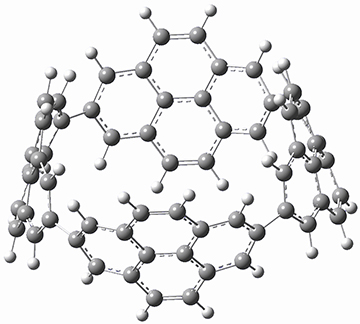
B3LYP/6-31G(d) optimization of the structure of 1 reveals a D2 geometry (Figure 1). This structure shows a very distorted pyrene unit. The strain energy of 1 is estimated as 392 kJ mol-1 (though how this was arrived at is not mentioned!), which is much larger than the strain energy of [8]-cycloparaphenylene.
Figure 1. B3LYP/6-31G(d) optimized structure of 1
This is another molecule to be sure to click on and rotate using JMol.
The nature of the HOMO and LUMO of 1 is very different than that of linear tetra-2,7-pyrene. The degenerate HOMOs and degenerate LUMOs of the linear compound have a node at the 2 and 7 positions and are localized to the terminal and central pyrene units, respectively. The HOMO and LUMO of 1 are fully delocalized. The implications of this are seen in the spectroscopy and electrochemistry of 1.
References
(1) Iwamoto, T.; Kayahara, E.; Yasuda, N.; Suzuki, T.; Yamago, S. "Synthesis, Characterization, and Properties of [4]Cyclo-2,7-pyrenylene: Effects of Cyclic Structure on the Electronic Properties of Pyrene Oligomers," Angew. Chem. Int. Ed. 2014, 53, 6430-6434, DOI: http://dx.doi.org/10.1002/anie.201403624.
InChIs
1: InChI=1S/C64H32/c1-2-34-18-50-20-36-4-3-35-19-49(17-33(1)57(35)58(34)36)51-21-37-5-7-41-25-53(26-42-8-6-38(22-51)59(37)61(41)42)55-29-45-13-15-47-31-56(32-48-16-14-46(30-55)63(45)64(47)48)54-27-43-11-9-39-23-52(50)24-40-10-12-44(28-54)62(43)60(39)40/h1-32H/b51-49-,52-50-,55-53-,56-54-
InChIKey=AWPUJIHMRRFHHU-GYSOBDCPSA-N