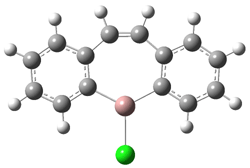
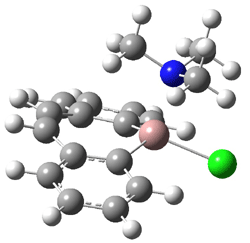
Robinson and Schleyer report the synthesis of and computations on the novel structure gallepin 1.1 This is the gallium analogue of tropyllium, the prototype of a seven-member aromatic ring. Robinson actually prepared the bis-benzannulated analogue 2, which is found to coordinate to TMEDA in the crystal.
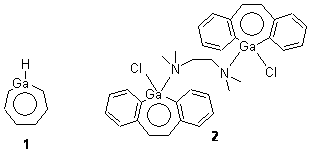
Schleyer computed (B3LYP/LANL2DZ) the gallepin portion of 2 in its naked form 3 and associated with trimethylamine 4. The crystal structure of 2 reveals that the 7 member ring is boat-shaped, and this is reproduced in the computed structure of 4. Interestingly, the naked gallepin is planar, suggestive of an aromatic structure. NICSπZZ computations were performed to gauge the aromaticity of these compounds. The value for the 7-member ring is -9.0 in 4 and -9.9 in 3, indicating aromatic character. These values are less then in the parent gallepin 1, which has a value of -15.3, but this is the normal type of diminishment expected from benzannulation.
But borapin has a NICSπZZ substantially more negative (-27.7) and so gallepins are less aromatic than borapins. Nonetheless, it is very interesting that aromaticity can be extended in this interesting way – different heteroatom and different ring size.
|
|
|
|
|
|
References
(1) Quillian, B.; Wang, Y.; Wei, P.; Wannere, C. S.; Schleyer, P. v. R.; Robinson, G. H., "Gallepins. Neutral Gallium Analogues of the Tropylium Ion: Synthesis, Structure, and Aromaticity," J. Am. Chem. Soc., 2007, 129, 13380-13381, DOI: 10.1021/ja075428d.