Poutsma has followed up on the work he reported earlier in collaboration with Kass concerning the gas-phase acidity of the amino acids.1 Their previous work reported on cysteine,2 with the unusual result that the thiol group is more acidic than the carboxylic acid group. (I blogged on this a previous post.) Now, he reports the experimental and DFT acidities of all 20 amino acids, shown in Table 1. The experiments were done using the kinetic method in a quadrupole ion trap with electrospray ionization. The computations were performed at B3LYP/6-311++G**//B3LYP/6-31+G*, following some MM searching to identify low-lying conformations. The computed acidities were obtained relative to acetic acid, i.e. R-CH2COOH + OAc– → R-CH2COO– +HOAc.
Table 1. Relative acidities (kJ mol-1) of the amino acids1
|
|
|
|
Exp |
DFT |
|
Gly (1434 ± 9) |
Gly (1434) |
|
Pro (1431 ± 9) |
Ala (1432) |
|
Val (1431 ± 8 ) |
Pro (1430) |
|
Ala (1430 ± 8 ) |
Val (1430) |
|
Ile (1423 ± 8 ) |
Leu (1428) |
|
Trp (1421 ± 9) |
Ile (1426) |
|
Leu (1419 ± 10) |
Trp (1422) |
|
Phe (1418 ± 18) |
Tyr (1419) |
|
Lys (1416 ± 7) |
Phe (1417) |
|
Tyr (1413 ± 11) |
Lys (1415) |
|
Met (1407 ± 9) |
Met (1412) |
|
Cys (1395 ± 9) |
Thr (1397) |
|
Ser (1391 ± 22) |
Cys (1396) |
|
Thr (1388 ± 10) |
Ser (1392) |
|
Asn (1385 ± 9) |
Arg (1387) |
|
Gln (1385 ± 11) |
Asn (1384) |
|
Arg (1381 ± 9) |
Gln (1378) |
|
His (1375 ± 8 ) |
His (1374) |
|
Glu (1348 ± 2) |
Glu (1349) |
|
Asp (1345 ± 14) |
Asp (1345) |
|
|
|
The computed values are in very good agreement with the experimental values. The amino acids are ordered in increasing acidity in Table 1. The order predicted by experiment and DFT are quite close, and the disagreements are well within the error bar of the experiment.
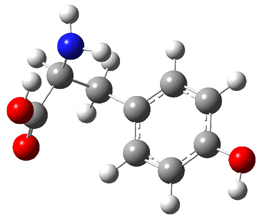
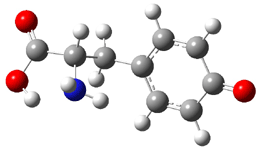
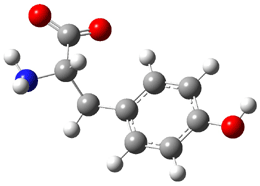
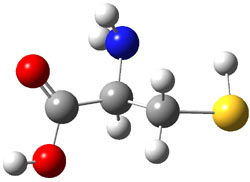
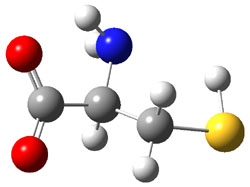
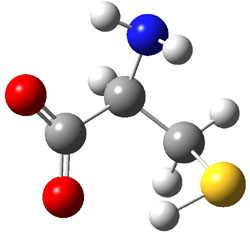
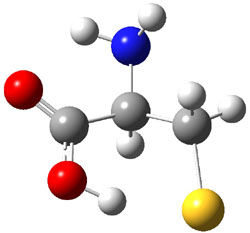
Similar to the result for cysteine, tyrosine also displays unusual acidity. The alcohol proton is more acidic than the carboxylic acid proton. The structures of tyrosine, and its two conjugate
bases, one from loss of the phenolic proton and the other from loss of the carboxylic acid proton are shown in Figure 1. The stability of the tyrosine conjugate base from loss of the phenolic
hydrogen arises from both the stability of phenoxide and the internal hydrogen bond from the carboxylic acid proton to the amine. This is different that in the cysteine case, the thiolate anion is stabilized by an internal hydrogen bond from the carboylic acid group (see Figure 2c here).
|
tyrosine |
|
|
Tyrosine conjugate |
Tyrosine conjugate |
Figure 1. B3LYP/6-31G* optimized structures of tyrosine and its conjugate bases.1
References
(1) Jones, C. M.; Bernier, M.; Carson, E.; Colyer, K. E.; Metz, R.; Pawlow, A.; Wischow, E. D.; Webb, I.; Andriole, E. J.; Poutsma, J. C., "Gas-Phase Acidities of the 20 Protein Amino Acids," Int. J. Mass Spectrom. 2007, 267, 54-62, DOI: 10.1016/j.ijms.2007.02.018.
(2) Tian, Z.; Pawlow, A.; Poutsma, J. C.; Kass, S. R., "Are Carboxyl Groups the Most Acidic Sites in Amino Acids? Gas-Phase Acidity, H/D Exchange Experiments, and Computations on Cysteine and Its Conjugate Base," J. Am. Chem. Soc. 2007, 129, 5403-5407, DOI: 10.1021/ja0666194.
InChI
Tyrosine: InChI=1/C9H11NO3/c10-8(9(12)13)5-6-1-3-7(11)4-2-6/h1-4,8,11H,5,10H2,(H,12,13)/t8-/m0/s1