Kass has once again uncovered a simple system that challenges our notions of basic chemical concepts. It is a well accepted notion that the most acidic proton of all of the amino acids is the carboxylic acid one. However, acidities are strongly influenced by the solvent, and the absence of solvent in the gas phase can dramatically alter things.
Kass and co-workers examined the gas-phase acidity of cysteine with computational and
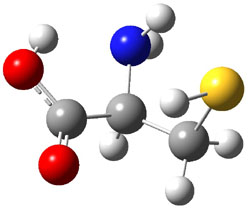
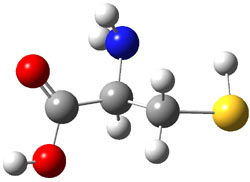
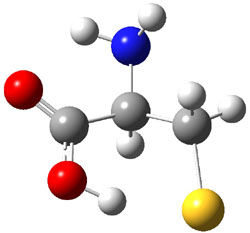
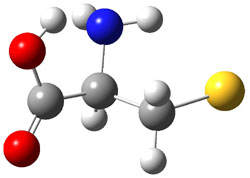
experimental techniques.1 The lowest energy conformer of cysteine is 1a, characterized by having three intramolecular hydrogen bonds (Figure 1). The next lowest conformer, 1b, has only two intramolecular hydrogen bonds and is 1.5 kcal mol-1 higher in energy at G3B3.
|
1a |
1b |
Figure 1. B3LYP/aug-cc-pVDZ optimized structures of cysteine 1.1
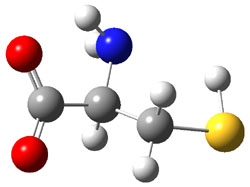
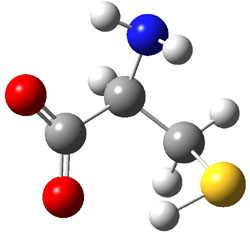
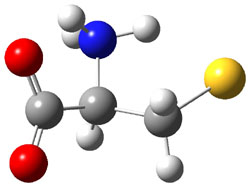
They optimized a number of different configurations of the conjugate base of cysteine: two conformers from the loss of the carboxylate proton (2a and 2b), two conformers from the loss of the thiol proton (2c and 2d), and one conformer from the loss of the thiol proton of the zwitterion (2e). These structures are shown in Figure 2 along with their relative energies. All of these structures possess two intramolecular hydrogen bonds.
|
2a |
2b |
|
2c |
2d |
|
|
|
|
2e |
|
Figure 2. B3LYP/aug-cc-pVDZ optimized structures of the conjugate base of cysteine 2. Relative energies (kcal mol-1) in parenthesis computed at G3B3.1
The gas phase acidity of carboxylic acids is greater than thiols; the deprotonation energy of propanoic acid (CH3CH2CO2H) is 347.7 kcal mol-1 at G3B3 (347.2 expt.2), about 6 kcal mol-1 less than that of ethanethiol (CH2CH2SH: 355.0 at G3B3 and 354.2 expt.2). However, the computations indicate that 2c is the lowest energy structure of deprotonated cysteine, and 2c comes about by loss of the thiol proton! Te lowest energy cysteine conjugate base from loss of the carboxylate proton is 1a, which is 3.1 kcal mol-1 higher in energy. Apparently, the hydrogen bonding network in 2c is quite favorable, able to make up for the inherent favorability of a carboxylate over a thiolate anion.
The G3B3 computed deprotonation energy of cysteine is 333.3 kcal mol-1 (for removal of the thiol proton). Kass determined the deprotonation energy of cysteine using a kinetic and a thermodynamic method. The kinetic method gives a value of 332.9 ± 3.3 kcal mol-1, while the thermodynamic method gives 334.4 ± 3.3 kcal mol-1. These are in fine agreement with the computed value.
This study ably demonstrates the dramatic role that solvent can play in determining molecular properties. Kass titled the article “Are carboxyl groups the most acidic sites in amino acids?” and answers with “no” – in the gas phase the thiol group is more acidic. He ends the article with an indication that the alcohol of tyrosine may be competitive in acidity with its carboxylic group, too.
References
(1) Tian, Z.; Pawlow, A.; Poutsma, J. C.; Kass, S. R., "Are Carboxyl Groups the Most Acidic Sites in Amino Acids? Gas-Phase Acidity, H/D Exchange Experiments, and Computations on Cysteine and Its Conjugate Base," J. Am. Chem. Soc., 2007, 129, 5403-5407, DOI: 10.1021/ja0666194.
(2) NIST, NIST Chemistry WebBook, 2005, http://webbook.nist.gov/.
InChIs
1: InChI=1/C3H7NO2S/c4-2(1-7)3(5)6/h2,7H,1,4H2,(H,5,6)

Computational Organic Chemistry » Amino acid acidity responded on 12 Nov 2007 at 7:27 am #
[…] Poutsma has followed up on the work he reported earlier in collaboration with Kass concerning the gas-phase acidity of the amino acids.1 Their previous work reported on cysteine,2 with the unusual result that the thiol group is more acidic than the carboxylic acid group. (I blogged on this a previous post.) Now, he reports the experimental and DFT acidities of all 20 amino acids, shown in Table 1. The experiments were done using the kinetic method in a quadrupole ion trap with electrospray ionization. The computations were performed at B3LYP/6-311++G**//B3LYP/6-31+G*, following some MM searching to identify low-lying conformations. The computed acidities were obtained relative to acetic acid, i.e. R-CH2COOH + OAc- → R-CH2COO- +HOAc. […]
Computational Organic Chemistry » Which is the Most Acidic Proton of Tyrosine? responded on 04 Mar 2009 at 11:16 am #
[…] Following on their prediction that the thiol of cysteine1 is more acidic than the carboxylic acid group (see this post), Kass has examined the acidity of tyrosine 1.2 Which is more acidic: the hydroxyl (leading to the phenoxide 2) or the carboxyl (leading to the carboxylate 3) proton? […]
Computational Organic Chemistry » Which is the Most Acidic Proton of Tyrosine? responded on 19 May 2009 at 8:05 am #
[…] prediction that the thiol of cysteine1 is more acidic than the carboxylic acid group (see this post), Kass has examined the acidity of tyrosine 1.2 Which is more acidic: the hydroxyl (leading to the […]