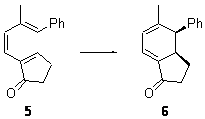
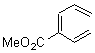While catalysis of many pericyclic reactions have been reported, until now there have been no reports of a catalyzed electrocylization. Bergman, Trauner and coworkers have now identified the use of an aluminum Lewis Acid to catalyze a 6e– electrocyclization.1
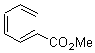
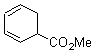
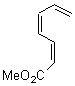
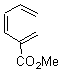
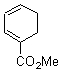
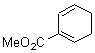
They start off by noting that electron withdrawing groups on the C2 position of a triene lowers the barrier of the electrocylization. So they model the carbomethoxy substituted hexatriene (1a-d) with a proton attached to the carbonyl oxygen as the Lewis acid at B3LYP/6-31G**. Table 1 presents the barrier for the four possible isomeric reactions. Only in the case where the substituent is in the 2 position is there a significant reduction in the activation barrier: 10 kcal mol-1.
Table 1. B3LYP/6-31G** activation barriers (kcal mol-1) for the catalyzed (H+) and uncatalyzed electrocylication reaction of carbomethoxy-substituted hexatrienes.
|
Reactant |
Product |
Ea |
Ea (protonated) |
|
|
|
31 |
35 |
|
|
|
34 |
33 |
|
|
|
24 |
14 |
|
|
|
26 |
24 |
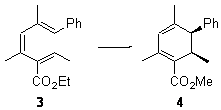
With these calculations as a guide, they synthesized compounds 3 and 5 and used Me2AlCl as the catalysts. In both cases, significant rate enhancement was observed. The thermodynamic parameters for these electrocylizations are given in Table 2. The aluminum catalyst acts primarily to lower the enthalpic barrier, as predicted by the DFT computations. The effect is not as dramatic as for the computations due likely to a much greater charge dispersal in over the aluminum catalyst (as opposed to the tiny proton in the computations) and the omission of solvent from the calculations.
Table 2. Experimental thermodynamic parameters for the electrocylcization of 3 and 5.
|
|
||
|
|
||
|
|
Thermal |
Catalyzed |
|
|
||
|
ΔH‡ (kcal mol-1) |
22.4 |
20.0 |
|
ΔS‡ (e.u.) |
-9.2 |
-11.8 |
|
ΔG‡ (kcal mol-1) |
25.2 |
23.5 |
|
|
||
|
|
||
|
|
Thermal |
Catalyzed |
|
|
||
|
ΔH‡ (kcal mol-1) |
20.3 |
18.1 |
|
ΔS‡ (e.u.) |
-12.4 |
-11.6 |
|
ΔG‡ (kcal mol-1) |
24.0 |
21.6 |
|
|
||
References
(1) Bishop, L. M.; Barbarow, J. E.; Bergman, R. G.; Trauner, D., "Catalysis of 6π Electrocyclizations," Angew. Chem. Int. Ed. 2008, 47, 8100-8103, DOI: 10.1002/anie.200803336
InChIs
1a: InChI=1/C8H10O2/c1-3-4-5-6-7-8(9)10-2/h3-7H,1H2,2H3/b5-4-,7-6+
InChIKey=INMLJEKOJCNLTL-SCFJQAPRBY
1b: InChI=1/C8H10O2/c1-3-4-5-6-7-8(9)10-2/h3-7H,1H2,2H3/b5-4-,7-6-
InChIKey=INMLJEKOJCNLTL-RZSVFLSABV
1c: InChI=1/C8H10O2/c1-4-5-6-7(2)8(9)10-3/h4-6H,1-2H2,3H3/b6-5-
InChIKey=QALYADPPGKOFPQ-WAYWQWQTBX
1d: InChI=1/C8H10O2/c1-4-6-7(5-2)8(9)10-3/h4-6H,1-2H2,3H3/b7-6+
InChIKey=BRDQFYBEWWPLFX-VOTSOKGWBR
2a: InChI=1/C8H10O2/c1-10-8(9)7-5-3-2-4-6-7/h2-5>,7H,6H2,1H3
InChIKey=LUFUPKXCMNRVLT-UHFFFAOYAU
2c: InChI=1/C8H10O2/c1-10-8(9)7-5-3-2-4-6-7/h2-3,5H,4,6H2,1H3
InChIKey=KPYYGHDMWKXJCE-UHFFFAOYAC
2d: InChI=1/C8H10O2/c1-10-8(9)7-5-3-2-4-6-7/h3,5-6H,2,4H2,1H3
InChIKey=YCTXQIVXFOMZCV-UHFFFAOYAU
3: InChI=1/C17H20O2/c1-5-16(17(18)19-4)14(3)11-13(2)12-15-9-7-6-8-10-15/h5-12H,1-4H3/b13-12+,14-11-,16-5-
InChIKey=DPBZDJWWRAWHQX-USTKDYFJBV
4: InChI=1/C17H20O2/c1-11-10-12(2)16(17(18)19-4)13(3)15(11)14-8-6-5-7-9-14/h5-10,13,15H,1-4H3/t13-,15-/m1/s1
InChIKey=JFBDOBRQORXZEY-UKRRQHHQBP
5: InChI=1/C16H16O/c1-13(12-14-6-3-2-4-7-14)10-11-15-8-5-9-16(15)17/h2-4,6-8,10-12H,5,9H2,1H3/b11-10-,13-12+
InChIKey=OAQIPONHGIZOBU-JPYSRSMKBG
6: InChI=1/C16H16O/c1-11-7-8-13-14(9-10-15(13)17)16(11)12-5-3-2-4-6-12/h2-8,14,16H,9-10H2,1H3/t14-,16-/m0/s1
InChIKey=MJUGKTLVECDOOO-HOCLYGCPBO