How would you characterized the benzotrithiophene 1? Is it planar? How about when methyl groups are attached (2)? Are these compounds aromatic? A joint computational/experimental study by Wu and Baldridge has tackled these questions.1

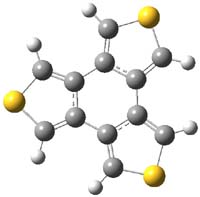
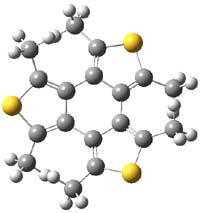
It turns out that both of these compounds are non-planar and have C2 symmetry. Now, 1 is very nearly planar. But 2 is decidedly non-planar. The MO6-2X/DZ(2d,p) structures are shown in Figure 1. The central 6-member ring has long bonds and expresses some bond alternation: the C-C distance for the bond between the thiophene rings is 1.469 Å and that of the bond shared by the two rings is 1.451 Å The exocyclic bonds are short, 1.375 Å. This appears to be [6]-radialene-like. NICS computations confirm this notion. The NICS(0) value for the central ring of 1 and 2 is -1.6, significantly less negative than the value in benzene of -7.2. The NICSzz values also reflect non-aromatic character of the central ring. The central ring is non-aromatic.
|
1 |
2 |
Figure 1. MO6-2X/DZ(2d,p) structures of 1 and 2.1
References
(1) Wu, T.-T.; Tai, C.-C.; Lin, W.-C.; Baldridge, K. K., "1,3,4,6,7,9-Hexamethylbenzo[1,2-c:3,4-c:5,6-c]trithiophene: a twisted heteroarene," Org. Biomol. Chem., 2009, 7, 2748-2755, DOI: 10.1039/b902517k.
InChIs
1: InChI=1/C12H6S3/c1-7-8(2-13-1)10-4-15-6-12(10)11-5-14-3-9(7)11/h1-6H
InChIKey=INZUTJPYADZZEL-UHFFFAOYAI
2: InChI=1/C18H18S3/c1-7-13-14(8(2)19-7)16-10(4)21-12(6)18(16)17-11(5)20-9(3)15(13)17/h1-6H3
InChIKey=WYVKJYPLPAEMLN-UHFFFAOYAA

Henry Rzepa responded on 24 May 2010 at 9:58 am #
William of Ockham (Occam) had the correct idea (entia non sunt multiplicanda praeter necessitatem). Try drawing a valence bond structure using a cyclohexatriene motif for the central ring. Only ionic forms are possible, and enforced charge separation is never entirely a good idea. Now try moving the S atom by one (i.e. rather than C-S-C, try C-C-S). I will bet, if that compound can be made, it will have a rather different structure!
Chemically, what is being reported here is the Clar effect, which states simply that the valence bond structure with the greatest number of benzenoid sextets is the most problable.
Sorry, I really do not think one always has to do a high level calculation to get insight! We will call this Hoffmann’s rule (Roald that is).