I blogged on Bickelhaput’s examination of the stability of kinked vs. linear polycyclic aromatics1 in this post. Bickelhaupt argued against any H…H stabilization across the bay region, a stabilization that Matta and Bader2 argued is present based on the fact that there is a bond path linking the two hydrogens.
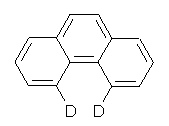
Grimme and Erker have now added to this story.3 They prepared the dideuterated phenanthrene 1 and obtained its IR and Raman spectra. The splitting of the symmetric (a1) and asymmetric (b1) vibrational frequencies is very small 9-12 cm-1. The computed splitting are in the same range, with very small variation with the computational methodology employed. The small splitting argues against any significant interaction between the two hydrogen (deuterium) atoms. Further, the sign of the coupling between the two vibrations indicates a repulsive interaction between the two atoms. These authors argue that the vibrational splitting is almost entirely due to conventional weak van der Waals interactions, and that there is no “bond” between the two atoms, despite the fact that a bond path connects them. This bond path results simply from two (electron density) basins forced to butt against each other by the geometry of the molecule as a whole.
|
|
References
(1) Poater, J.; Visser, R.; Sola, M.; Bickelhaupt, F. M., "Polycyclic Benzenoids: Why Kinked is More Stable than Straight," J. Org. Chem. 2007, 72, 1134-1142, DOI: 10.1021/jo061637p
(2) Matta, C. F.; Hernández-Trujillo, J.; Tang, T.-H.; Bader, R. F. W., "Hydrogen-Hydrogen Bonding: A Stabilizing Interaction in Molecules and Crystals," Chem. Eur. J. 2003, 9, 1940-1951, DOI: 10.1002/chem.200204626
(3) Grimme, S.; Mück-Lichtenfeld, C.; Erker, G.; Kehr, G.; Wang, H.; Beckers, H. W., H., "When Do Interacting Atoms Form a Chemical Bond? Spectroscopic Measurements and Theoretical Analyses of Dideuteriophenanthrene," Angew. Chem. Int. Ed. 2009, 48, 2592-2595, DOI: 10.1002/anie.200805751
InChIs
1: InChI=1/C14H10/c1-3-7-13-11(5-1)9-10-12-6-2-4-8-14(12)13/h1-10H/i7D,8D
InChIKey=YNPNZTXNASCQKK-QTQOOCSTEC

Henry Rzepa responded on 20 May 2009 at 11:45 am #
The controversy over H…H interactions is replicated for F…F interactions. With all those lone pairs on fluorine, it is even more unreasonable to expect those two atoms to form a bond, and yet when they are forced into proximity, that is what the AIM methods suggests happens. This was reported by Boyd (10.1016/j.cplett.2005.04.088) who found that the new F…F bond then completed a helical turn, creating two ring points and a cage point as well. So the ramifications of such bond points can be substantial.
Mind you, the ELF procedures, which replace the ρ(r) function with an electron localization function, have their own foibles. In this scheme, a bond is characterized by a synaptic basin, within which an electron density can be integrated to give an electron count for the bond. The weaker bonds often have no such basin. So when is a bond an interaction? Do interactions mean as much as bonds? Clearly a controversial topic!
Henry Rzepa responded on 03 Feb 2010 at 1:37 am #
I have found an interesting new angle on this problem. In a blog post I reported a conformational analysis of cyclo-octane. One of the stable minima for this species has D2d symmetry, in which two pairs of hydrogens approach to around 1.9Å. The two CH vibrations involved do not mix with the others (i.e.there is no need to deuterate to removing mixing with other modes), one being, symmetric the other anti-symmetric. I calculated the anharmonic values, speculating that the two modes would have different anharmonic corrections depending on whether the H…H region was attractive or repulsive. Go see the result for yourself at my blog!