Bickelhaupt has reported a broad benchmark study of the prototype SN2 and E2 reactions.1 These are the reactions of ethyl fluoride with fluoride and ethyl chloride with chloride (Scheme A). The critical points were optimized at OLYP/TZ2P and then CCSD(T)/CBS energies are used as benchmark. A variety of different density functionals were then used to obtain single-point energies.
Scheme A
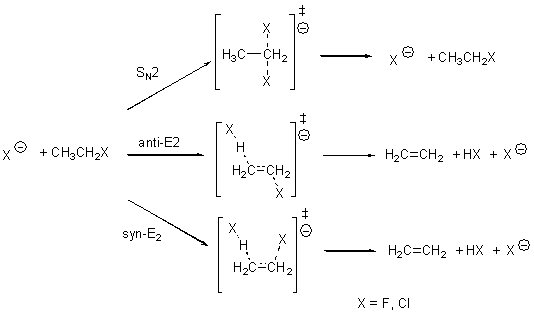
The relative energies of the transition states for the six different reactions are listed for some of the functionals in Table 1. (These are energies relative to separated reactants – and keep in mind that an ion dipole complex is formed between the reactants and the transition states – Bickelhaupt calls this a “reaction complex”.)
Table 1. Relative energies (kcal mol-1) of the transition states for the six reactions shown in Scheme A.
|
|
||||||
|
|
F- |
Cl- |
||||
|
Method |
SN2 |
E2 anti |
E2 syn |
SN2 |
E2 anti |
E2 syn |
|
CCSD(T) |
2.20 |
-1.27 |
5.68 |
5.81 |
18.18 |
30.92 |
|
BLYP |
-11.27 |
-11.55 |
-8.66 |
-3.69 |
5.28 |
14.04 |
|
PW91 |
-11.39 |
-9.58 |
-9.29 |
-3.24 |
6.38 |
14.22 |
|
PBE |
-10.73 |
-9.36 |
-8.98 |
-2.43 |
6.85 |
14.75 |
|
B3LYP |
0.24 |
-5.38 |
-2.00 |
0.92 |
11.00 |
21.22 |
|
MO5-2X |
3.97 |
0.99 |
3.85 |
6.84 |
12.58 |
28.46 |
|
MO6-2X |
5.82 |
1.49 |
4.03 |
10.73 |
10.65 |
30.29 |
|
|
||||||
There is a lot more data in this paper, along with a summary of the mean absolute errors in the overall and central barriers that mimics the data I show in Table 1. The trends are pretty clear. Generalized gradient approximation (GGA) functions – like BLYP, PW91, and PBE – dramatically underestimate the barriers. The hybrid functionals perform much better. The recently maligned B3LYP functional gets the correct trend and provides reasonable estimates of the barriers. Truhlar’s MO5-2X and MO6-2X functionals do very well in matching up the barrier heights along with getting the correct trends in the relative barriers. Simply looking for the functional with the lowest absolute error is not sufficient; BHandH and MO6-L have small errors but give a wrong trend in barriers, predicting that the SN2 reaction is preferred over the E2 for the fluoride reaction.
Reference
(1) Bento, A. P.; Sola, M.; Bickelhaupt, F. M., "E2 and SN2 Reactions of X– + CH3CH2X (X = F, Cl); an ab Initio and DFT Benchmark Study," J. Chem. Theory Comput., 2008, 4, 929-940, DOI: 10.1021/ct700318e.
