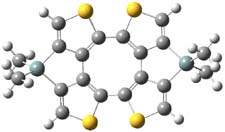
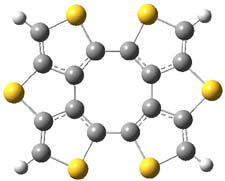
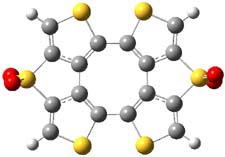
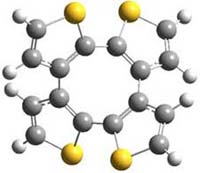
Here’s another attempt (almost successful!) in creating a planar cyclooctatetraene. Nishininaga and Iyoda have fused silicon and sulfur bridges to the COT framework, hoping to force the 8-member ring out of its preferred tub-shape into a planar structure.1 They report the synthesis of 1, 2, and 3b along with their x-ray structures. They also calculated the structures at B3LYP/6-31G(d,p) for 1-4 , and these optimized structures are shown in Figure 1.

|
1 |
2 |
|
3a |
4 |
Figure 1. B3LYP/6-31G(d,p) optimized geometries of 1-4. The experimental (top) and computed (Bottom in italics) value of α are listed for each compound.1
The bent angle α is defined at the angle between the two planes that define the bottom of the tub and one of the sides. For COT itself, this angle is 40°, decidedly non-planar – as expected for a molecule avoiding the antiaromatic character it would have in its planar conformation. The computed and experimental values of α are shown in Figure 1. 4 is tub shaped. The value of α for 1 is about 18° – still tub shaped but flattened. But 2 and 3 are nearly planar, with experimental values of α about 3° and the computed values are similar.
So what is the character of the 8-member ring in these compounds. The computed NICS(0) values are 3.8 ppm for 4, the expected small value for a non-aromatic compound. (Note that the NICS value for COT is 2.9 ppm.) The values are much more positive for the other compounds: 12.7 ppm for 1, 17.4 ppm for 2, and 15.4 ppm for 3a. These compounds therefore display antiaromatic character yet they are isolable compounds!
References
(1) Ohmae, T.; Nishinaga, T.; Wu, M.; Iyoda, M., "Cyclic Tetrathiophenes Planarized by Silicon and Sulfur Bridges Bearing Antiaromatic Cyclooctatetraene Core: Syntheses, Structures, and Properties," J. Am. Chem. Soc., 2009, 132, 1066-1074, DOI: 10.1021/ja908161r
InChIs
1: InChI=1/C20H16S4Si2/c1-25(2)9-5-21-17-13(9)14-10(25)6-22-18(14)20-16-12(8-24-20)26(3,4)11-7-23-19(17)15(11)16/h5-8H,1-4H3/b19-17-,20-18-
InChIKey=DAVVQYAXJVCICC-CLFAGFIQBV
2:
3a: InChI=1/C16H4O4S6/c17-25(18)5-1-21-13-9(5)10-6(25)2-23-15(10)16-12-8(4-24-16)26(19,20)7-3-22-14(13)11(7)12/h1-4H/b14-13-,16-15-
InChIKey=VIDZCGPUBZEACC-RFIZXXDFBR
3b: InChI=1/C28H36O4S6Si4/c1-39(2,3)25-21-13-14-19(35-26(40(4,5)6)22(14)37(21,29)30)20-16-15-18(17(13)33-25)34-27(41(7,8)9)23(15)38(31,32)24(16)28(36-20)42(10,11)12/h1-12H3/b18-17-,20-19-
InChIKey=XXCFCYWSFICMIO-RXGVRZIVBS
4: InChI=1/C16H8S4/c1-5-17-13-9(1)10-2-6-18-14(10)16-12(4-8-20-16)11-3-7-19-15(11)13/h1-8H/b10-9-,12-11-,15-13-,16-14-
InChIKey=RSNUTSCZGMAXQJ-FNJUYVFOBD

Henry Rzepa responded on 17 Mar 2010 at 8:44 am #
Did I just imagine the tangible surprise in your last statement These compounds therefore display antiaromatic character yet they are isolable compounds?
In fact, lots of antiaromatic compounds are not merely stable, but crystalline, with X-ray structure determinations. Many expanded porphyrins are anti-aromatic, a various other larger cycles. I blogged about one of my own favourites, not least because the people who discovered it did not seem to realize quite what they had made.
So yes, the oxymoronic stable antiaromatics are here to stay. Has anyone reviewed the area?
Steven Bachrach responded on 17 Mar 2010 at 8:53 am #
I am not surprised that this is an isolable antiaromatic compound. I am aware of your work, and others (my colleague here at Trinity University Nancy Mills has spend decades synthesizing these beasts), who have predicted and made these seemingly self-contradictory molecules – and have blogged on them too. I was simply emphasizing that this seeming self-contradiction is in fact not a contradiction.
I am not familiar with a review of stable antiaromatics, but would welcome any pointers.
Henry Rzepa responded on 17 Mar 2010 at 10:56 am #
Not perhaps entirely self-contradictory. I vaguely recollect, in the 1930s and the Hückel era, the first calculation (not by Hückel) which revealed that cyclo-octatetraene WAS resonance stabilized, just not nearly as much as benzene (the same paper contained a comprehensive analysis of the relative energies of boat and chair forms of cyclohexane, years before Barton et al made it fashionable as conformational analysis). I think Paul Schleyer has may have shown that planar COT is resonance stabilised more recently.