The standard model for explaining enzyme activation is that the active site is designed to stabilize the transition state, thereby reducing the activation barrier. Jonathan Goodman offers a very compelling argument for an alternative explanation for at least some enzymes.1
He examined enzymes that coordinate the substrate through what’s called an “oxyanion hole”, a region in the active site where an incipient oxyanion can be stabilized through 2 or three hydrogen bonds. This usually involves nucleophilic attack at a carbonyl. Analysis of the protein data bank turned up several hundred such structures where a carbonyl is coordinated to the enzyme by 2 or more hydrogen bonds. Also examined were several hundred small molecule x-ray structures that also exhibit this sort of hydrogen bonding scheme. The geometry about the carbonyl oxygen was examined – distances angles and dihedral angles – and the only significant difference between the enzyme and small molecule set is for the dihedral angle formed between the O=C-R plane of the carbonyl and the C=O…H angle to the hydrogen bond donor. For the small molecules, the preferred value is about 0°, but for the enzymes, the preferred angle is about 90°.
MPWB1K/6-311++G**//B3LYP/6-31G(d,p) computations of a model enzyme active site (see Scheme 1) were performed where the two waters are arranged at different dihedral angles. For both reactant and transition state, the coordinating waters stabilize the structures – and there is a stabilization for all dihedral angles.
Scheme 1
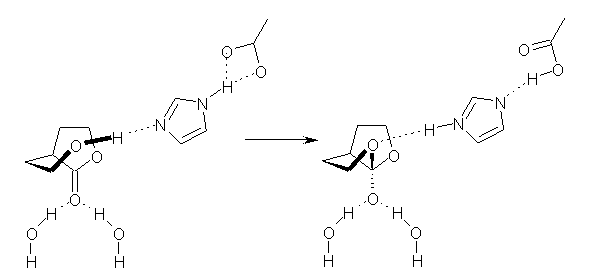
But the best arrangement, i.e. the maximum stabilization, occurs when the waters are arranged with a dihedral angle of 0° for both the reactant and transition state. At 0°, the reactant is significantly stabilized, more so than the stabilization of the TS. At 90° stabilization of both species is less than at 0° but the stabilization is much less for the reactant than for the TS. Thus, at 90° the activation barrier is lowered not by preferential stabilization of the TS but by lesser stabilization of the reactant! The active site is set up not to stabilize the TS but rather to minimize the activation barrier through differential stabilization of the reactant vs the TS. This new model offer another approach towards creating artificial catalysts, ones designed not to maximize binging, but rather to minimize the activation barrier through judicious stabilization of the TS and destabilization of the reactant.
References
(1) Simon, L.; Goodman, J. M., "Enzyme Catalysis by Hydrogen Bonds: The Balance between Transition State Binding and Substrate Binding in Oxyanion Holes," J. Org. Chem. 2010, DOI: 10.1021/jo901503d

Henry Rzepa responded on 23 Jan 2010 at 2:11 pm #
This is a fascinating result, not least because it focuses attention on a simple aspect of bonding, what is the spatial orientation of lone pairs in common arrangements such as e.g. the carbonyl group. There is a tendency to simplify the answer to such questions; thus in alcohols and ethers, the lone pairs on oxygen are supposedly tetrahedrally arranged around the heteroatom; in carbonyls they are co-planar with the R-C=O plane, etc. The former arrangement for example explains most anomeric effects.
But our use of the ELF technique, which is very good at identifying the centroids of the monosynaptic basins which most lone pairs occupy, has revealed a variety of surprises. One such, which I found today, and which has in fact prompted this comment, was for Zeise’s salt (ethene.PtCl3)(-). One might imagine that the chlorine lone pairs (each nominally has three) would not be of particular interest, but far from it!
It is the nature of the WordPress system which Steve is using here that one cannot post pictures (or 3D coordinates) in a comment, and so I will perhaps frustrate the reader by leaving it at that! Maybe I might become impelled to describe the oddity on my own blog.
But my message here is that nowadays, one need not have to assume a simple model in attributing orientation to a lone pair (and hence the hydrogen bonds that result). It remains to be established whether ELF is truly more useful than these simple models, but I suggest that if one does thus explore, some surprises may emerge!