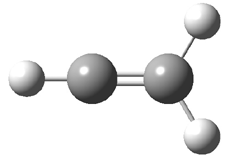
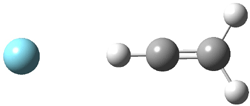
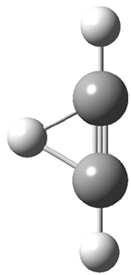
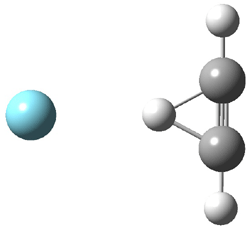
Duncan and Schleyer1 have investigated protonated acetylene and the protonated acetylene dimer. These ions are created in a pulsed supersonic nozzle/pulsed electrical discharge with a weakly bound argon atom as a tag. IR laser photodissociation spectroscopy allows for the detection of peaks down to 2000 cm-1, a region not previously explored for this cation. The experimental IR spectrum for H+(C2H2).Ar has two main features: at 3146 and 2217 cm-1. The 3146 cm-1 corresponds to the previously observed peak2 at 3142 cm-1 and is similar to the absorption in acetylene (3136 cm-1). MP2/6-311+G(2d,2p) computations were performed on the classical and non-classical structures of H+(C2H2), with and without a complexed argon atom. These geometries are displayed in Figure 1 and the predicted vibrational frequencies are listed in Table 1.
|
|
|
|
|
|
Figure 1. MP2/6-311+ Table 1. Relative energies (kcal mol-1) and frequencies of protonated acetylene Rel E Frequencies (scaled) H+(acetylene)Ar non-classical 0.0 3139, 2123 H+(acetylene)Ar classical 7.8 3084, 2954, 2878, 1673 H+(acetylene) non-classical 0.0 3219, 2250 H+(acetylene) classical 7.1 3162, 29947, 2874 Experiment 3364, 3212, 3146, 2217 The argon tag only slightly perturbs the spectrum, as expected for a weakly bond atom remote from most of the hydrogen atoms. The predicted spectra of the two non-classical ions are in nice agreement with the experiment – particularly the interesting peak at 2123 cm-1 that is due to the bridged proton. This spectra, and the confirmation of the bridging, non-classical structure, makes a nice pair with the recently reported bridging, non-classical structure of the ethyl cation,3 which I blogged on previously. The spectrum of the H+(C4H4) ion show a doublet at 3129 and 3158 cm-1 and two small peaks at 1261 and 1365 cm-1. The computed structure that comes closest to matching this spectrum is for the asymmetrically bridged dimer (See Figure 2), though is much more energetic than its isomers. The authors speculate that the bridged dimer is trapped in an energy-well during the thermal expansion, which prevents the formation of the lower energy isomers. Figure 2. Schematic drawing and relative energies of the H+(C4H4) ion. (1) Douberly, G. E.; Ricks, A. M.; Ticknor, B. W.; McKee, W. (2) Gabrys, C. M.; Uy, D.; Jagod, M. F.; Oka, T.; Amano, T., "Infrared Spectroscopy of Carboions. 8. Hollow Cathode Spectroscopy of Protonated Acetylene, C2H3+," J. Phys. Chem., 1995, 99, 15611-15623, DOI: 10.1021/j100042a042. (3) Andrei, H.-S.; Solcà, N.; Dopfer, O., "IR Spectrum of the Ethyl Cation: Evidence
and the protonated acetylene-argon cluster.1
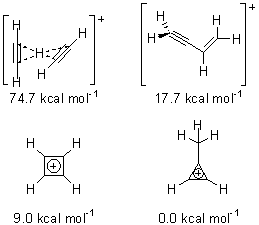
(Note – unfortunately the authors have supplied insufficient information in the Supporting Materials to completely define the geometries of these molecules!)References
C.; Schleyer, P. v. R.; Duncan, M. A., "Infrared Photodissociation
Spectroscopy of Protonated Acetylene and Its Clusters," J. Phys. Chem. A, 2008, 112, 1897-1906, DOI: 10.1021/jp710808e.
for the Nonclassical Structure," Angew. Chem. Int. Ed., 2008, 47, 395-397, DOI: 10.1002/anie.200704163