The photochemistry of m-xylylene 2 has been studied by Sander1 and, as might be anticipated, it’s fascinating! Flash vapor pyrolysis of 1 produces 2. Photolysis of 2 at wavelengths above 400nm gives 3 and 4, while photolysis at 254 nm gives 5. These are products are novel strained hydrocarbons. Confirmation of their structures was obtained by comparing their experimental IR spectra with that computed at B3LYP/6-311G(d,p). Table 1 compares the experimental and computed IR absorptions for 2-5. Note in particular the fine agreement between the two, especially the predicted changes due to i>d4 substitution for all the phenyl positions.

Table 1. Experimental and Calculated vibrational frequencies (cm-1) of 2-5.1
|
|
||||
|
Mode |
ν (expt) |
ν (calc) |
ν (expt) |
ν (calc) |
|
|
2 |
2-d4 |
||
|
11 |
640.5 |
655.0 |
645.6 |
661.3 |
|
12 |
723.9 |
721.0 |
581.0 |
576.5 |
|
15 |
766.4 |
777.0 |
759.1 |
772.6 |
|
16 |
834.9 |
849.0 |
831.4 |
847.3 |
|
|
3 |
3-d4 |
||
|
11 |
733.6 |
757.8 |
681.1 |
698.8 |
|
16 |
869.9 |
899.9 |
808.1 |
822.2 |
|
17 |
883.2 |
915.3 |
703.4 |
726.5 |
|
33 |
1640.6 |
1696.4 |
1614.4 |
1658.8 |
|
|
4 |
4-d4 |
||
|
10 |
706.6 |
715.8 |
627.1 |
633.9 |
|
11 |
757.4 |
770.8 |
689.7 |
702.3 |
|
15 |
874.2 |
896.6 |
763.5 |
784.2 |
|
23 |
1065.2 |
1082.7 |
1001.4 |
1017.5 |
|
|
5 |
5-d4 |
||
|
10 |
742.9 |
764.2 |
660.5 |
676.4 |
|
16 |
851.7 |
876.4 |
787.2 |
797.5 |
|
17 |
852.9 |
880.1 |
789.8 |
799.9 |
|
33 |
1678.6 |
1747.1 |
166.2 |
1709.7 |
|
34 |
1683.8 |
1758.7 |
1669.2 |
1719.9 |
|
|
||||
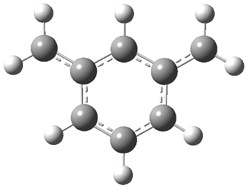
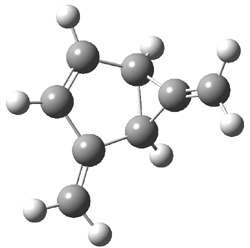
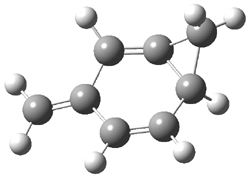
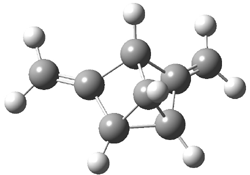
The computed structures of 2-5 and their relative energies are shown in Figure 1. Triplet 1 is the lowest energy isomer, with the singlet-triplet gap of 6.22 kcal mol-1. This compares with recent high-level computations which give a value of 13.8 kcal mol-1. 2 The other structures are much higher in energy. These other isomers have unusual bonding environments – 3 contains the strained methylenecyclopropane group, 4 is an anti-Bredt compound, and 5 is a very strained tricycle. These compounds can only be prepared by the application of light to provide the energy needed for their creation.
|
2 |
3 |
|
4 |
5 |
Figure 1. B3LYP/6-311G(d,p) optimized structures of 2-5 and their relative energies (kcal mol-1).1
References
(1) Neuhaus, P.; Grote, D.; Sander, W., "Matrix Isolation, Spectroscopic Characterization, and Photoisomerization of m-Xylylene," J. Am. Chem. Soc., 2008, 130, 2993-3000, DOI: 10.1021/ja073453d.
(2) Wang, T.; Krylov, A. I., "The effect of substituents on electronic states’ ordering in meta-xylylene diradicals: Qualitative insights from quantitative studies," J. Chem. Phys., 2005, 123, 104304, DOI: 10.1063/1.2018645.
InChIs
2: InChI=1/C8H8/c1-7-4-3-5-8(2)6-7/h3-6H
3: InChI=1/C8H8/c1-5-3-4-7-6(2)8(5)7/h3-4,7-8H,1-2H2; InChIKey=IAJQBWKVTDKBHW-UHFFFAOYAA
4: InChI=1/C8H8/c1-6-2-3-7-5-8(7)4-6/h2-4,7H,1,5H2; InChIKey=XSFFUTQMPAAWHN-UHFFFAOYAU
5: InChI=1/C8H8/c1-3-5-4(2)7-6(3)8(5)7/h5-8H,1-2H2; InChIKey=USZJYFGTTGREFL-UHFFFAOYAB

Justin Davies responded on 10 Apr 2008 at 4:07 am #
Hi, It would be really good if you started adding ResearchBlogging tags to your posts. If you haven’t been already, take a look at http://www.researchblogging.org/