Birney has published another study of a pseudopericyclic reaction to complement the many I discus in Chapter 3.4. Here he looks at that decarbonylation of benzothiophenedione 1, which if analogous to the furandione will first give the ketene 2 before forming 3.1 Upon gentle heating, 3 dimerizes to 4. Interestingly, 2 has not been detected.2

2 does not exist as a local minimum on the B3LYP/6-31G(d,p) or G3MP2B3 surfaces; rather all optimizations collapse to 3. However, when optimized with PCM with the dielectric of DMSO, 2 is a local minimum.
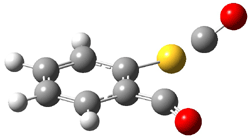
The transition state for loss of CO from 1 leads directly to 3. This TS (TS1-3, see Figure 1) is non-planar, unlike for the analogous reaction of the furandione. TS1-3 does not correspond with a pseudopericyclic reaction.
|
TS1-3 |
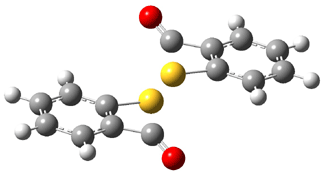
TS3-4 |
Figure 1. B3LYP/6-31G(d,p) optimized geometries of TS1-3 andTS3-4.1
The transition state for the dimerization of 3 (TS3-4), also shown in Figure 1, appears to be a [2σs + σs] cyclization, which is thermally forbidden. However, analysis of the molecular orbitals indicates the interaction of sets of orthogonal orbitals, exemplary of a pseudopericyclic reaction. The barrier for this reaction, 17.9 kcal mol-1, is consistent with an allowed pseudopericyclic process.
References
(1) Sadasivam, D. V.; Birney, D. M., "A Computational Study of the Formation and Dimerization of Benzothiet-2-one," Org. Lett., 2008, 10, 245-248, DOI: 10.1021/ol702628v.
(2) Wentrup, C.; Bender, H.; Gross, G., "Benzothiet-2-ones: Synthesis, Reactions, and Comparison with Benzoxet-2-ones and Benzazetin-2-ones," J. Org. Chem., 1987, 52, 3838-3847, DOI: 10.1021/jo00226a022.
InChIs
1: InChI=1/C8H4O2S/c9-7-5-3-1-2-4-6(5)11-8(7)10/h1-4H
2: InChI=1/C7H4OS/c8-5-6-3-1-2-4-7(6)9/h1-4H
3: InChI=1/C7H4OS/c8-7-5-3-1-2-4-6(5)9-7/h1-4H
4: InChI=1/C14H8O2S2/c15-13-9-5-1-3-7-11(9)17-14(16)10-6-2-4-8-12(10)18-13/h1-8H