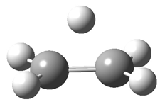
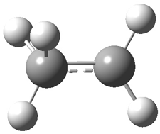
The structure of the simple, fundamental ethyl cation has finally been ascertained. Computational studies had long suggested the non-classical structure 1 for this cation. The classical structure 2 is a transition state for scrambling the protons. The MP2/6-311G(2d,p) geometries of both structures are shown in Figure 1.
|
1 |
2 |
|
1.Ar(C2v) |
1.Ar(Cs) |
Figure 1. MP2/6-311G(2d,f) structures of 1, 2, 1.Ar(C2v) and 1.Ar(Cs).
Dopfer1 has now obtained IR spectrum of ethyl cation by single-photon IR photodissociation spectroscopy through the reaction
C2H5+ . Ar + hν → C2H5+ + Ar
Two structures of the ethyl cation associated with Ar were optimized at MP2/6-311G(2df,2pd). (The MP2/6-311G(2d,p) structures are shown in Figure 1.) Both of their computed IR spectra have stretches at nearly identical wavenumbers as for ethyl cation 1 itself. The experimental IR spectra has absorptions at 3317 and 3037 cm-1, very close to the computed frequencies for 1.Ar(C2v). This provides strong experimental evidence that ethyl cation is in fact a non-classical ion.
References
(1) Andrei, H.-S.; Solcà, N.; Dopfer, O., "IR Spectrum of the Ethyl Cation: Evidence for the Nonclassical Structure," Angew. Chem. Int. Ed. 2008, 47, 395-397, DOI: 10.1002/anie.200704163
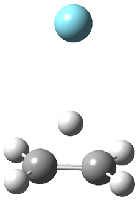
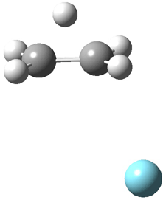

Henry Rzepa responded on 29 Feb 2008 at 2:34 am #
Ethyl is not the only such species whose structure is not certain. As students, we are all taught that the bromonium ion is an essential in the mechanism for bromination of an alkene. But it seems that alkenes other than ethene probably exist in a classical rather than cyclic form. Perhaps Dopfer can perform his experiments on this species as well?
Computational Organic Chemistry » Protonated acetylene responded on 01 May 2008 at 9:52 am #
[…] The argon tag only slightly perturbs the spectrum, as expected for a weakly bond atom remote from most of the hydrogen atoms. The predicted spectra of the two non-classical ions are in nice agreement with the experiment – particularly the interesting peak at 2123 cm-1 that is due to the bridged proton. This spectra, and the confirmation of the bridging, non-classical structure, makes a nice pair with the recently reported bridging, non-classical structure of the ethyl cation,3 which I blogged on previously. […]
Network Theory (Part 14) « Azimuth responded on 14 Oct 2011 at 9:13 pm #
[…] • Stephen Bacharach, Ethyl cation, Computational Organic Chemistry. […]