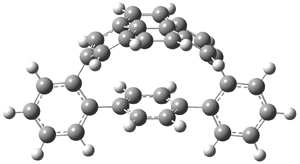
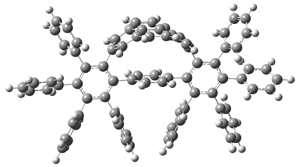
The novel cyclophanes 1 and 2 have now been synthesized.1 An interesting question is whether the bent pyrenes portion of the two molecules remains aromatic. The bending angles is 93.8° in 1 and 95.8° in 2. This distortion is readily apparent in Figure 1, which presents their B3LYP/6-311G(d,p) optimized geometries. NICS computations were used to assess the aromaticity of the pyrene portion. The central rings of pyrene have NICS(0) = -4.4 ppm. The corresponding values in 1 and 2 are -4.5 ppm. The apical rings of pyrene have NICS(0)= -11.9 ppm, while the value is -11.1 ppm in 1 and -11.0 ppm in 2. These calculations indicate that the molecule retains much of the aromaticity of the parent pyrene despite the significant out-of-plane distortions.
Figure 1. B3LYP/6-311G(d,p) optimized geometries of 1 and 2.1
|
1 |
2 |
References
(1) Zhang, B.; Manning, G. P.; Dobrowolski, M. A.; Cyranski, M. K.; Bodwell, G. J., "Nonplanar Aromatic Compounds. 9. Synthesis, Structure, and Aromaticity of 1:2,13:14-Dibenzo[2]paracyclo[2](2,7)-pyrenophane-1,13-diene," Org. Lett., 2008, 10, 273-276, DOI: 10.1021/ol702703b.
InChIs
1: InChI=1/C34H20/c1-3-7-31-27-17-23-13-15-25-19-28(20-26-16-14-24(18-27)33(23)34(25)26)32-8-4-2-6-30(32)22-11-9-21(10-12-22)29(31)5-1/h1-20H/b29-21-,30-22-,31-27-,32-28-
InChIKey=RYJKEGNHXVXOPX-LISLPQAZBN
2: InChI=1/C82H52/c1-9-25-53(26-10-1)71-73(55-29-13-3-14-30-55)77(59-37-21-7-22-38-59)81-67-49-63-45-47-65-51-68(52-66-48-46-64(50-67)69(63)70(65)66)82-78(60-39-23-8-24-40-60)74(56-31-15-4-16-32-56)72(54-27-11-2-12-28-54)76(58-35-19-6-20-36-58)80(82)62-43-41-61(42-44-62)79(81)75(71)57-33-17-5-18-34-57/h1-52H/b79-61-,80-62-,81-67-,82-68-
InChIKey=BKSFSTJXYFPKOG-IPILNRTDBN

ChemSpiderMan responded on 14 Feb 2008 at 7:16 pm #
Steven, I watch your pages and add structures of interest to ChemSpider. The power of the InChIKey connectivity is clear in this example.
Visit http://www.chemspider.com/Chemical-Structure.21105586.html and click on the InChIKey in the middle of the page and you will find one hit…this page of yours. Thanks for the great story. I’m off to add the second structure 🙂
sbachrach responded on 15 Feb 2008 at 9:22 am #
Tony,
Thanks for the link. The ChemSpider page on this molecule is very useful – especially because of the annotations you have made.
I have slowly been adding in InChIKeys (I use the ChemSpider service for generating these, by the way!). I am still unsure as to the proper/standard way to include InChIkeys within web docs and am hoping for some guidance here at some point.