What happens when antiaromatic rings stack? One can draw an MO interaction diagram for π-stacked cyclobutadiene dimer (Figure 1) and recognize at once that this cluster should be stabilized. In fact, it is reminiscent of an orbital diagram for an aromatic species!

Figure 1. MO Interaction diagram of stacked butadiene (modified from Ref 1).
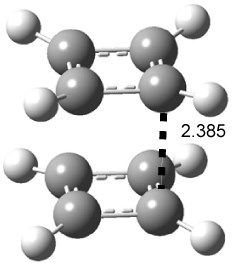
Houk had examined just this dimer (1) in 1996 and located a D4h critical point at CASSCF(8,8)/6-31G* (see Figure 2).2 This structure is energetically below two isolated cyclobutadiene molecules; however, it is a second-order saddle point.
1
Figure 1. CASSCF(8,8)/6-31G* optimized structure of 1.
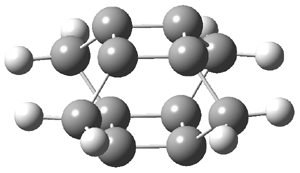
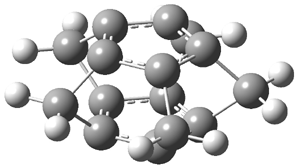
Schleyer has examined a series of superphanes constructed from anti- and aromatic rings linked by methano bridges, 2-7.1 These structures were optimized at B3LYP/6-311+G** and their magnetic properties computed at GIAO-PW91. The optimized structures of 3 and 4 are shown in Figure 3.
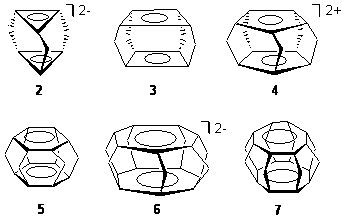
|
3 |
4 |
Figure 3. B3LYP/6-311+G** optimized structures of 3 and 4.1
The inter-ring separation (D) in these compounds is quite interesting (Table 1). It decreases in the series 2-4, with the distance in the latter compound of only 2.002 Å. The inter-ring distance is much larger in 5, which has two (aromatic) benzene rings. All of the other comounds (except 2) have shorter distances and these all involve antiaromatic rings. These short distances for the antiaromatic superphanes suggests stabilizing interactions between the rings, as indicated by the MO diagram of Figure 1.
Table 1. Inter-ring distance and NICS values for 2-7.1
|
|
|||
|
Compound |
Da |
NICScage |
NICS(1)zzring |
|
2 |
2.365 |
-47.9 |
-15.3 |
|
3 |
2.055 |
-41.6 |
-7.6 |
|
4 |
2.002 |
-46.7 |
-9.2 |
|
5 |
2.305 |
-8.1 |
-7.4 |
|
6 |
2.202 |
-29.8 |
-17.0 |
|
7 |
2.162 |
-35.5 |
-21.8 |
|
|
|||
aDistance (Å) between the carbon of one ring and the closest carbon of the second ring.
The NICS values are also interesting. Schleyer computed a variety of different NICS values, and we list here the isotropic NICS value at the cage center (NICScage) and the zz-component evaluated 1 Å above the ring on the outside face NICS(1)zzring). The NICS(1)zzring is perhaps the best measure of magnetic properties related to aromatic/antiaromatic character. All six compounds have rings that have negative values of NICS(1)zzring, indicating of aromatic character. In fact, the value for 5 is less negative than for isolated benzene alone. This suggests that the stacked antiaromatic rings become aromatic, while the stacked aromatic rings become less aromatic. For all six compounds, the NICScage value is negative indicating diatropicity, associated with aromatic character – again consistent with the MO argument presented in Figure 1. To answer our lead off question, stacked antiaromatic rings are aromatic!
References
(1) Corminboeuf, C.; Schleyer, P. v. R.; Warner, P., "Are Antiaromatic Rings Stacked Face-to-Face Aromatic?," Org. Lett. 2007, 9, 3263-3266, DOI: 10.1021/ol071183y.
(2) Li, Y.; Houk, K. N., "The Dimerization of Cyclobutadiene. An ab Initio CASSCF Theoretical Study," J. Am. Chem. Soc. 1996, 118, 880-885, DOI: 10.1021/ja921663m.
InChIs
2: InChI=1/C9H6/c1-4-6-2-7-5(1)9(7)3-8(4)6/h1-3H2/q-2
3: InChI=1/C12H8/c1-5-7-2-8-6(1)10-3-9(5)11(7)4-12(8)10/h1-4H2
4: InChI=1/C15H10/c1-6-8-2-9-7(1)11-3-10(6)14-5-15(11)13(9)4-12(8)14/h1-5H2/q+2
5: InChI=1/C18H12/c1-7-9-2-10-8(1)12-3-11(7)15-5-16(12)18-6-17(15)13(9)4-14(10)18/h1-6H2
6: InChI=1/C21H14/c1-8-10-2-11-9(1)13-3-12(8)16-5-17(13)21-7-20(16)18-6-19(21)15(11)4-14(10)18/h1-7H2/q-2
7: InChI=1/C24H16/c1-9-11-2-12-10(1)14-3-13(9)17-5-18(14)22-7-21(17)23-8-24(22)20-6-19(23)15(11)4-16(12)20/h1-8H2