In Chapter 5.3.2, I extensively discuss the organocatalyzed aldol reaction. Barbas and List have pioneered the use of proline to catalyze this reaction, and Houk has performed a series of computational studies to discern the mechanism. The mechanism is essentially the attack of the enamine on the carbonyl with concomitant proton transfer from the carboxylic acid to the forming oxyanion.
Shininisha and Sunoj have examined a number of bicyclic analogues of proline (1-11) as catalysts of the aldol reaction.1 They computed the activation energies for the reaction of the enamine derived from acetone with p-nitrobenzaldehyde with the various catalysts. All computations were performed at B3LYP/6-311+G**//B3LYP/6-31G* with the solvent effects modeled using CPCM.

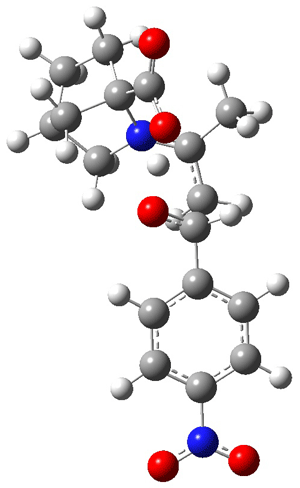
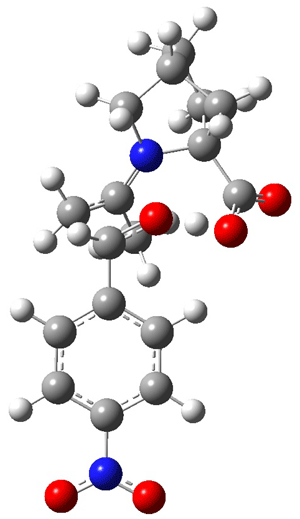
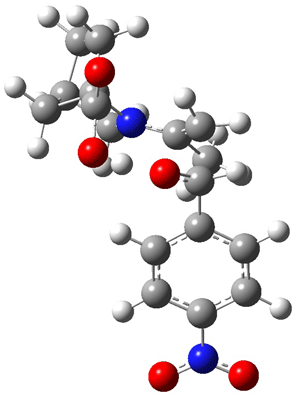
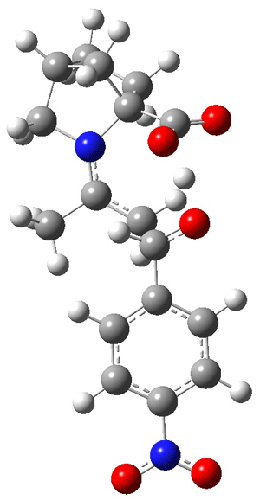
As Houk has demonstrated, there are four possible transition states: the attack can come to either the re or si face of the aldehyde and either the syn or anti enamine can be the reactant. The four transition states for the reaction of 8 are shown in Figure 1. These TSs are representative of all of the transition states involving the different catalysts, including proline itself. These TS are characterized by proton transfer accompanying the C-C bond formation. Their relative energies can be interpreted in terms of the distortions about the enamine double bond (the more planar, the lower the energy) and the arrangement of the carboxylic acid and the incipient oxyanion. These arguments were made by Houk and are described in my book.
|
8-anti-re |
8-anti-si |
|
8-syn-re |
8-syn-si |
Figure 1. B3LYP/6-311+G**//B3LYP/6-31G* optimized structures and relative energies (kcal/mol) of the transition states of the enamine derived from acetone and 8 with p-nitrobenzaldehyde1
The enantiomeric excess predicted by the computations for the aldol reaction using the 11 different bicyclic catalysts is presented in Table 1. All of the catalysts except 11 give high enantiomeric excess, with a number of them predicted to produce an ee above 90%. The authors conclude that these catalysts are worth exploring, since they are predicted to perform better than proline (which has a predicted ee of 75%).
Table 1. Predicted ee for the reaction of the enamine derived
from acetone and catalyst with p-nitrobenzaldehyde.
|
|
|
|
Catalyst |
ee |
|
1 |
87 |
|
2 |
85 |
|
3 |
82 |
|
4 |
91 |
|
5 |
92 |
|
6 |
90 |
|
7 |
84 |
|
8 |
95 |
|
9 |
75 |
|
10 |
80 |
|
11 |
5 |
|
|
|
References
(1) Shinisha, C. B.; Sunoj, R. B., "Bicyclic Proline Analogues as Organocatalysts for Stereoselective Aldol Reactions: an in silico DFT Study," Org. Biomol. Chem., 2007, 5, 1287-1294, DOI: 10.1039/b701688c.
InChIs
1: InChI=1/C8H13NO2/c1-5-4-6-2-3-8(5,9-6)7(10)11/h5-6,9H,2-4H2,1H3,(H,10,11)
2: InChI=1/C8H13NO2/c1-5-4-8(7(10)11)3-2-6(5)9-8/h5-6,9H,2-4H2,1H3,(H,10,11)
3: InChI=1/C6H9NO2/c8-5(9)6-2-1-4(3-6)7-6/h4,7H,1-3H2,(H,8,9)
4: InChI=1/C6H9NO3/c8-5(9)6-2-1-4(7-6)10-3-6/h4
5: InChI=1/C5H7NO3/c7-4(8)5-1-3(6-5)9-2-5/h3,6H,1-2H2,(H,7,8)
6: InChI=1/C6H9NO2S/c8-5(9)6-2-1-4(7-6)10-3-6/h4,7H,1-3H2,(H,8,9)
7: InChI=1/C5H7NO2S/c7-4(8)5-1-3(6-5)9-2-5/h3,6H,1-2H2,(H,7,8)
8: InChI=1/C7H11NO2/c9-6(10)7-2-1-5(3-7)4-8-7/h5,8H,1-4H2,(H,9,10)
9: InChI=1/C7H11NO2/c9-7(10)6-4-1-2-5(3-4)8-6/h4-6,8H,1-3H2,(H,9,10)
10: InChI=1/C6H9NO2/c8-6(9)5-3-1-4(2-3)7-5/h3-5,7H,1-2H2,(H,8,9)
11: InChI=1/C6H9NO2/c8-6(9)5-3-1-4(5)7-2-3/h3-5,7H,1-2H2,(H,8,9)