The norbornyl cation has been a source of controversy for decades. Just what is the nature of this cation? Should one consider it a classical cation A or of some non-classical character B? A recent computational study adds further fuel to this fire.1

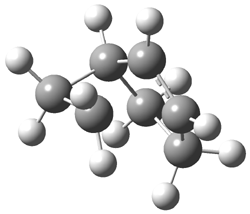
The B3LYP/6-311G(d,p) structure of the norbornyl cation is shown in Figure 1, and this structure is little changed when reoptimized at PBE1PBE/6-311G(d,p) or CCSD/6-311G(d,p). Application of the topological method (sometimes referred to as atoms-in-molecules or AIM) reveals a bond path network that resembles the bicyclo[3.2.0]heptyl cation C. The C1-C2 distance is 1.75 Å and a bond path does connect these two atoms, though the density at the bond critical point is only 60% the value at the other C-C bonds in the compound. There is no bond path connecting C1 to C3 that would close up a three-member ring. The C1-C3 distance is 1.955 Å. So, the non-classical structure is not a proper description of this unusual species.
Figure 1. B3LYP/6-311G(d,p) optimized structure of the norbornyl cation.
References
(1) Werstiuk, N. H., "7-Norbornyl Cation – Fact or Fiction? A QTAIM-DI-VISAB Computational Study," J. Chem. Theory Comput., 2007, 3, 2258-2267, DOI: 10.1021/ct700176d.

Henry Rzepa responded on 26 Feb 2008 at 11:03 am #
The norbornyl cation has fascinated many people for many years, and many schemes have been proposed to describe it. AIM analysis, in some people’s opinions, is biased towards 2-center interactions, and almost always reduces a 3-center interaction such as this one to two centers (any 3-center solution which is found by imposing symmetry is unstable, as noted in Werstiuk’s article). Another insight however is obtained by studying a phenomenon known as homoaromaticity. See for example http://dx.doi.org/10.1021/jp026521l which is an AIM analysis conducted using ELF rather than electron density functions. Here, it is recognized that many homoaromatics (all from positive ions) show NO bond critical points! Despite that, the centers are clearly interacting. So AIM is predisposed to NOT revealing bond critical points in such (electron deficient) systems.
I would like to suggest here an unusual (original?) description of the norbornyl and related cations. It is a no-bond homoaromatic, with one bridging bond being a homoaromatic bond, the other conventional σ. Two (equivalent) such structures are possible. The interpretation of norbornyl as (nobond)homoaromatic is yet another way of considering this issue; it is of course a 4n+2 aromatic, following the Huckel rule of aromaticity (n=0). This augments the non-classical σ interpretation, and Dewar’s π-complex theory.
And in an effort to stir up more controversy, might I suggest that the conventional picture of diborane exhibiting 3-center-2-electron bonds is also wrong. An AIM/ELF interpretation is of a double agostic interaction between two BH3 units. This is NOT quite equivalent to saying its a 3-center-two-electron interaction, which in itself derives from (non unique) MO pictures rather than electron density topologies.